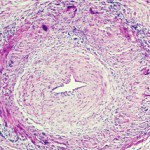
Inflammatory infiltrates in the media and adventitia have different composition. T cells are dominant in these lesions, and these T cells may produce IFN-γ, IL-17, IL-21, IL-9, IL-2 and IL-22, a highly diverse effector cell population that suggests multiple antigens are driving disease, “because so many different inflammatory cells are driving the lesion,” said Dr. Weyand. “What the lesion looks like from an immunological perspective is like it is unopposed, or to use a more modern term, like the immune response is ‘unleashed.’ So the question is how can this unleashed immune response get into a site that is normally protected by immune privilege—a site that nature had determined it does not want to have any inflammatory activity for any price?”
Research findings show three failures in this immune privilege in GCA: Patients have increases in CD4 T cells, a leakiness in the aorta’s access portal that would normally keep out inflammatory cells and defective immune checkpoints.
In their lymphoid tissues, GCA patients have low levels of CD8 T-regulatory cells with a surface marker called NADPH oxidase. Normally, macrophages use this oxidase to form exosomes that infiltrate CD4 cells and stop their function, killing bacteria. Immune dysregulation in GCA causes these patients to have more active CD4 T cells, she said.1
Inflammatory cells enter the aorta through the vasa vasoral networks of the adventitia. Using biopsy data, she and her colleagues found two signals of gene expression in the inflamed arteries of GCA patients: Jagged-1 and NOTCH-1.2 Jagged-1 is the ligand and NOTCH-1 is the receptor, she said. Through high-resolution imaging, they found that Jagged-1 is expressed on the vasa vasorum structures, and the ligand is expressed on the cell surface. With flow cytometry, they also found that the receptor sits on the circulating CD4 T cells of GCA patients.
“We see that patients with GCA have a population of NOTCH-positive cells. If we look in these cells, they have a signal for a protein called HES, which is NOTCH-signaling dependent. Every one of these NOTCH cells is transmitting that signal,” she said. They also found that GCA patients have increased vascular endothelial growth factor (VEGF) that upregulates Jagged-1 on the microvascular cells. Through studies on specially bred mice, the researchers confirmed that both Jagged-1 and NOTCH-1 signaling are VEGF dependent. VEGF increases interferon production in CD3 T cells found in GCA lesions, she said. VEGF may be blocked with axitinib.
Tocilizumab may help GCA patients lower their overall steroid dose & decrease relapse risk.
Checkpoint Breakdown
As in cancer, immune checkpoint function is important in GCA pathogenesis. Tumors evade immune systems by producing negative, inhibitory ligands that stop T cells sent to kill those tumors. In GCA, patients have an immune checkpoint deficiency, said Dr. Weyand. They have low levels of the inhibitory ligand PD-L1 in their inflamed temporal arteries and high levels of the receptor, PD-1.