Just as a rising tide lifts all boats, the Rheumatology Informatics System for Effectiveness (RISE) registry lifts all rheumatology research. Developed by the ACR, RISE initially arose from federal reporting requirements.
Although the initial role of RISE was to help clinicians navigate the changing payment landscape and track quality of care, it has also become a vast repository of unique data on millions of rheumatology patients from across the U.S.
RISE at a Glance
“We’re proud to say the RISE registry is the first and largest national EHR-enabled rheumatology registry in the country, with more than 30% of U.S. rheumatologists participating,” says Rachel Myslinski, vice president of practice, advocacy & quality for the ACR.
The RISE data set is a unique treasure trove, with data on more than 1.5 million patients representing about 17 million encounters. “The information in the RISE registry is real-world data collected during the routine course of clinical care,” says Tracy Johansson, the ACR’s director of registry analytics. “In short, it represents what clinicians are actually doing in their practices.”
How to Access the Data for Research
Researchers interested in using RISE data for research can submit requests through the ACR’s online request form. All requests are reviewed by ACR staff and the ACR’s Research and Publications Subcommittee, a group of volunteers familiar with RISE data and experienced in using EHR data for research.
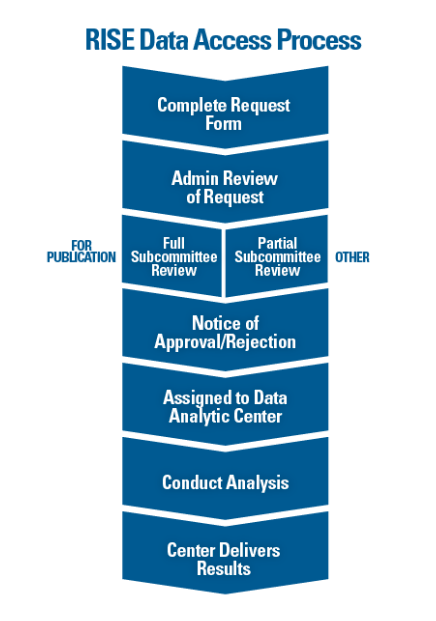 Katherine Liao, MD, MPH, chair of the ACR’s Research and Publications Subcommittee, says the review process is focused on feasibility (ensuring the questions posed can be answered by RISE data) and preventing overlap of projects. However, subcommittee members can provide valuable feedback. “Individuals who sit on the subcommittee are all funded investigators. Each person knows how to think deeply about pertinent research questions, and we all have our fingers on the pulse of the rheumatology community.”
Katherine Liao, MD, MPH, chair of the ACR’s Research and Publications Subcommittee, says the review process is focused on feasibility (ensuring the questions posed can be answered by RISE data) and preventing overlap of projects. However, subcommittee members can provide valuable feedback. “Individuals who sit on the subcommittee are all funded investigators. Each person knows how to think deeply about pertinent research questions, and we all have our fingers on the pulse of the rheumatology community.”
The review process is iterative, allowing requestors to make updates to their proposal as they see fit based on the feedback they receive at each stage. Once a request is approved, the project is assigned to one of three RISE data analytic centers (DACs)—University of California, San Francisco (UCSF), University of Alabama, Birmingham, and Duke Clinical Research Institute—each of which is led by a rheumatology expert.
To be clear, researchers do not have direct access to the data. “The database is not set up so an investigator can actually touch the data,” says Dr. Liao. “The data must be cleaned and maintained, and we must be able to ensure confidentiality. This means that requestors work very closely with the team at the assigned DAC.”
Researchers are responsible for covering the costs associated with their request. So what is the price for using RISE data? “Project fees are determined based on the complexity of each project, translating into effort,” says Dr. Liao. That means the cost ranges widely. “A variety of pathways [are available] to assist requestors,” she continues.
Gabriela Schmajuk, MD, is the rheumatology lead at the UCSF Data Analytic Center. Her center worked on several abstracts being presented at the 2019 ACR/ARP Annual Meeting. She says the investment in RISE data presents a rare opportunity for researchers. “RISE is a unique resource because it automatically extracts and aggregates all EHR data from participating practices. The data, which primarily come from small practices, cover a segment of rheumatology care that is not often studied because most rheumatology data emanate from academic centers.”
The treasure trove that is RISE data will continue to grow. Jinoos Yazdany, MD, MPH, chair of the ACR Registries and Health Information Technology Committee since 2017, says efforts are underway to build the capacity to conduct more advanced analytic projects. “The first stage was getting the data from over 300 sites and dozens of different EHR systems clean, validated and organized for research. Now we are moving into advanced analytics, whereby we will be able to take this big data and use techniques, such as artificial intelligence and natural language processing, to extract and study richer clinical concepts. I am excited to be working with the incredibly dedicated RISE team, all of whom share a vision of the future of rheumatology and what it takes to advance the evidence base in our field.”
The ACR is also working to expand the RISE data set through the incorporation of claims data, such as Medicare.
Drs. Yazdany, Schmajuk and Liao will share more details and answer questions about the RISE registry, the process to request RISE data for research and the discoveries being made with RISE data in a session at the 2019 ACR/ARP Annual Meeting on Wednesday, Nov. 13, Using the RISE Registry to Improve Practice & Research.
Real-World Research
The breadth of research that can be done using RISE data will also be on display at the 2019 ACR/ARP Annual Meeting in Atlanta, with nine abstract presentations scheduled.
Zara Izadi, a PhD candidate in epidemiology at UCSF, will present her study, “The ACR’s Rheumatology Informatics System for Effectiveness (RISE) Demonstrates Improvements in Many Measures of Quality of Care between 2015 and 2017.” The study examined changes in performance on eight quality measures among dozens of RISE practices over a two-year period.
Another study, “ANCA-Associated Vasculitis Management in the United States: Data from the RISE Registry,” will be presented by Zachary Wallace, MD, MSc, a rheumatologist at Massachusetts General Hospital in Boston. His project focused on evaluating treatment patterns for ANCA-associated vasculitis. Dr. Wallace notes the advantages of being able to use such a unique data set: “We’ve seen that RISE can be used to study the variety of ways patients with rheumatoid arthritis are actually being treated,” he says. “Now we’re seeing that it also provides an incredible opportunity to study less common rheumatic diseases, like vasculitis, where sample sizes from other data sets would be too small.”
Dr. Wallace explains that his work with the RISE data analytic center at the University of Alabama, Birmingham, was collaborative and focused on fulfilling his research goals.
Ms. Izadi worked with the RISE DAC at UCSF on her project and says the analytics team demonstrated expertise and was “prompt and very thorough in running the queries for me.”
RISE at the Annual Meeting
Abstracts using data from RISE that will be presented at the 2019 ACR/ARP Annual Meeting include:
- Identifying Comorbidities and Seropositivity in Rheumatoid Arthritis Patients Using Single-specialty Electronic Health Record Data (poster, Nov. 10, 9–11 a.m.)
- Characteristics of Patients with Systemic Sclerosis in the Rheumatology Informatics System for Effectiveness (RISE) Registry (poster, Nov. 10, 9–11 a.m.)
- Pre-treatment Screening for Hepatitis B and C Among Users of Biologics or New Synthetic Disease Modifying Drugs: An Analysis Using RISE Data (poster, Nov. 10, 9–11 a.m.)
- The ACR’s Rheumatology Informatics System for Effectiveness (RISE) Demonstrates Improvements in Many Measures of Quality of Care between 2015 and 2017 (poster, Nov. 10, 9–11 a.m.)
- Patterns of Newer Gout Medication Use in a U.S. Electronic Health Record-Based Registry (poster, Nov. 10, 9–11 a.m.)
- ANCA-Associated Vasculitis Management in the United States: Data from the RISE Registry (oral presentation, Nov. 10, 2:30–4 p.m.)
- Patterns of Medication Use for Patients with Sarcoidosis: Data from the ACR’s RISE Registry (poster, Nov. 11, 9–11 a.m.)
- Treatment Response to Biologic DMARDs in Patients with RA: A Retrospective Analysis of the RISE Registry (poster, Nov. 11, 9–11 a.m.)
- Gaps in Care for Patients with SLE: Data from the ACR’s RISE Registry (oral presentation, Nov. 10, 4:30–6 p.m.)
Sessions featuring information on RISE data for research purposes at the 2019 ACR/ARP Annual Meeting include:
- Premeeting Clinical Research Conference: Big Data: Analytics, EMR, Registries and Beyond (paid session, Nov. 9, 7 a.m.–4:15 p.m.)
- Using the RISE Registry to Improve Practice & Research (session, Nov. 13, 9–10 a.m.)
Elizabeth Hofheinz, MPH, MEd, is a freelance medical editor and writer based in the greater New Orleans area.