1) Is nomenclature and classification based on vessel size possibly misleading or an oversimplification of vascular biology? For example, within those illnesses described as “small vessel vasculitides,” not all small vessels share the same vulnerability. Indeed, vasculitis rarely occurs in the microvasculature of the pancreas, adrenal glands, thyroid, tongue, or retina. However, it is relatively common in the skin, lungs, and kidneys.
Among those diseases we call “large vessel vasculitis,” territories of large vessels may differ markedly in their vulnerability to injury. It is more common to find lesions in the proximal than in the distal aorta and in the branch vessels of the arch versus those of the distal aorta.
Our sense of intellectual comfort about understanding the pathogenesis of vasculitis is further compromised by examples of diseases thought to be exclusively small- or medium-sized vessel occasionally affecting the aorta (e.g., systemic lupus erythematosus and Sjögren’s syndrome vasculitis) or, conversely, giant cell arteritis affecting the skin or retinal vessels. What is the basis for this selective vulnerability beyond vessel size?
2) While rare, single-organ vasculitis is a well-known entity that is often cured by surgical resection of the affected component or the entire organ when feasible (e.g., testicle, breast, and gynecologic organ). These examples of presumed limited autoimmunity would theoretically be simpler to study than complex multiorgan vasculitides, and findings may improve our understanding of systemic vasculitis. In what manner are these entities similar to other single-organ immune-mediated diseases (e.g., type I diabetes, hypo- and hyperthyroidism, Addison disease, multiple sclerosis, and primary biliary cirrhosis)?
In many of these examples, abnormal immune responses have been identified to unique single-organ antigens such as insulin, myelin oligodendrocyte glycoprotein, thyrotropin receptor, thyroid peroxidase, and thyroglobulin. Are these immune aberrations to organ-specific proteins proximal, distal, or secondary and inconsequential in pathogenesis? In systemic vasculitides, might shared antigens or homologous antigens in multiple tissues determine multifocal targeting?
3) Is the immune response in each organ unique to that site in some fashion? Are different organs better equipped than others to avoid injury or clear pathogens without resultant harm to the site? Might this in part explain “organ selectivity” which indeed may not be a matter of pathogen tropism?
These questions have begun to generate answers, and the results are extremely exciting. The subsequent discussion will focus on the following issues:
- Early development and modification of vessels during embryogenesis and subsequent life;
- The striking differences that exist between vessels of the same size in different organs, as well as differences that exist even in the proximal and distal territories of the same vessel; and
- Unique immunologic profiles of different vessels and their implied susceptibility or resistance to pathogens.
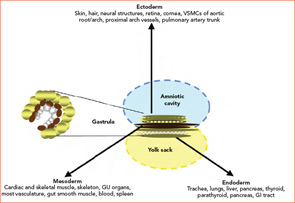
Embryogenesis
During the third week of human embryonic development, the progenitors of individual organ systems are already present. Undifferentiated cells have become ectoderm, mesoderm, and endoderm. These three layers and their derivatives are represented in Figure 1 (above). The genetic program in nascent organs unfolds in a fashion that is not only unique in regard to the specialized roles of formative parenchyma but also unique to the vasculature and other constituent structures (e.g. nerve, matrix, and lymphatics). The genetic program will change over time, and the products of developing cells may in turn influence the development of neighboring cells. Embryologists speak of these events as “context dependent.” The results may vary depending on context: time and place.