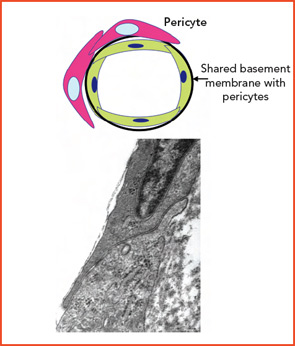
Diversity among small-vessel ECs extends beyond the cells themselves. Microvascular channels within different organs may be supported by surrounding matrix components that differ both qualitatively or quantitatively (e.g., including within vascular basement membranes: collagens, nidogens, laminins, fibronectin, and fibrillin). Adjacent ECs in different organs may function in a profoundly different manner based on physical and physiological relationships with basement membrane and surrounding cells (e.g., pericytes, SMCs, organ parenchymal cells).5,6 (See Figures 2A and 2B, p. 17.)
These relationships form the basis for selectivity of transvascular passage of both cells and chemicals. Where passage is most permissive is within the discontinuous (sinusoidal) endothelium of the liver; it is most selective within the continuous endothelium of the retina and brain. Consistent with the known plasticity of vascular biology, especially during early development, are changes that may occur to adapt to needs such as the early brain containing fenestrated ECs and later the more mature brain having a continuous EC microvascular bed. From a broad range of data that spans macro to microvascular, physical properties to genetic and molecular arrays, it is clear that vascular territories and their component cellular and matrix elements are as unique as the organs they perfuse.
Age-Related Change
The aging process is associated with fragmentation of elastic fibers, depletion of elastica, and an increase in collagen deposition. The most significant changes occur in conjunction with an increase in vessel-wall concentrations of matrix metalloproteinases in muscular/elastic vessels. Coupling of vessel and tissue proteins with reducing sugars leads to production of advanced glycation end products that alter the function of normal proteins and may change their antigenicity. Evidence of progressive EC dysfunction includes a reduced capacity to produce nitric oxide and an increased engagement of pathways of oxidative stress that enhance risk of atherosclerosis and thrombosis. These changes may vary in severity in different sites.8-10
Over our lifespans, we experience innumerable interactions with our environment, acquiring, in some cases, inanimate materials by transcutaneous, airborne, or ingested pathways. Other acquisitions include living materials that were transmitted as early as during gestation (e.g., microchimerisms, or cells from our mothers), or later from others or the environment (e.g., infectious agents, especially herpes viruses). Each may have favorite locations within tissue, including vessels, where they may be of no consequence or could lead to overt infection or changes in tissue antigen expression.
Immunologic Susceptibility
In a seminal study, the laboratory of Cornelia Weyand and Jorg Goronzy extended its prior observations, identifying and characterizing Toll-like receptors (TLRs) in temporal arteries, particularly at the adventitial–medial borders. They have now asked the question of whether or not disease patterns may be related to unique innate immune profiles within specific vascular regions. They compared expression of TLRs 1–9 in the thoracic aorta, carotid, subclavian, temporal, mesenteric, and iliac arteries.11 Each artery was found to possess a unique TLR portfolio. TLRs 2 and 4 were consistently present in all arteries studied; TLRs 7 and 9 were infrequent; and the greatest variability was noted for TLRs 1, 3, 5, 6, and 8. Using their well-known SCID chimera model, they were able to demonstrate that lipopolysaccharide (LPS)-stimulated mice infused with CD 4+ lymphocytes could establish an effective adaptive immune response, whereas controls (unstimulated) did not. If dendritic cells had been depleted by stripping the adventitia from the SCID-embedded vessels, CD4 cells did not home to the vessel. This work established that adventitial–medial dendritic cells are critical to pathogen “sensing” and that the ability of muscular arteries to achieve this goal varies between vessels from different territories.