Wegener’s granulomatosis (WG) is a complex multisystem vasculitic disease of unknown cause. Although once rapidly progressive and often fatal, WG is now a manageable condition in which remission can be achieved by conventional immunosuppressive therapy. With the introduction of effective therapy by Fauci and Wolff in the 1970s and 1980s, the dreaded nature of this disease was dramatically changed as investigators gained new insights into the pathogenesis and course of WG. Importantly, they had the opportunity to understand the relapsing nature of WG, appreciate the toxicities of therapy, and explore the basis for the granuloma formation and vasculitis. In 2008, it is not unusual to see people with WG who have had their illness for many years. Their course today reflects both the success made as well as the progress we have yet to make.
A Case in Point
Consider the case of a 55-year-old female who, in 1991, was diagnosed with WG involving the sinuses and lungs. She was treated initially with prednisone and daily cyclophosphamide (CYC). As was the standard of care at that time, she received CYC for one year, which was then tapered and discontinued. She did well off medications for 36 months and then had a relapse involving her sinuses, peripheral nerves, and kidneys, with a peak creatinine of 2.0 mg/dL. She again received prednisone and CYC, and her creatinine normalized. After three months of treatment, she had gross hematuria, with cystoscopy revealing CYC-induced bladder injury. She was switched to azathioprine (AZA), on which she did well. After two years, while still on AZA, she experienced a sinus and lung relapse. She was taken off of AZA and treated with prednisone and methotrexate (MTX). Her disease improved but was complicated by diabetes mellitus. Over the next five years, she continued to experience sinonasal and joint relapses, for which she was kept on MTX with adjustments in her prednisone dose. She is currently in remission on MTX, but she remains diabetic and has residual sinus symptoms and neuropathic foot pain. Despite the ups and downs of her condition, she continues to enjoy her work, family, and community activities.
Clinical Features
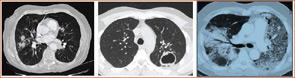
WG is most commonly associated with disease of the sinuses, lungs, and kidneys, but it is very much a multisystem disease.1 Other sites of involvement include the skin, joints, eyes, and peripheral or central nervous system. Pulmonary involvement can have many radiographic presentations, including single or bilateral nodules or infiltrates, cavities, or ground glass infiltrates suggestive of alveolar hemorrhage (see Figure 2, p. 21). Subglottic and endobronchial stenosis should be considered in any WG patient with an unexplained cough or dyspnea. Ninety percent of patients with WG will first present with upper and or lower airway symptoms. Because asymptomatic disease can occur in the lungs and kidneys, pulmonary imaging and urinalysis should be performed in all patients when WG is being considered. Patients with WG have an increased incidence of venous thrombotic events, making awareness of deep venous thrombosis or pulmonary embolism important.2
The diagnosis of WG is typically confirmed by biopsy of the affected organs. As the histologic changes are patchy, the diagnostic yield is associated with the biopsy location and amount of tissue obtained. Surgical biopsies of radiographically abnormal pulmonary parenchyma have a yield of 90%, but diagnostic features are found in fewer than 10% of transbronchial biopsies.
Breakthrough: Antineutrophil Cytoplasmic Antibodies
The discovery of antineutrophil cytoplasmic antibodies (ANCA) opened many clinical and investigational avenues in WG. Antibodies directed against proteinase-3 (PR-3) produce a cytoplasmic staining pattern (cANCA) by immunoflurorescence. These types of antibodies occur in 75% to 90% of patients with WG, and 5% to 20% of patients have anti-myeloperoxidase (MPO) antibodies that have a perinuclear staining pattern (pANCA).3
Because WG is a rare disease, the utility of ANCA testing for diagnosis depends upon the site of organ involvement. In patients with sinus, lung, and renal disease, a (+) PR3-ANCA has a predictive value of over 90% for WG. However, the predictive value is only 30% to 60% in patients with sinus and lung disease, making biopsy confirmation important in this setting to rule out infection or neoplasm.
WG is not the only vasculitic disease in which ANCA occur. MPO-ANCA are seen in 50% to 80% of patients with microscopic polyangiitis, with 10% to 20% having PR3-ANCA. ANCA are also found in up to 40% of patients with Churg-Strauss syndrome, where anti-MPO ANCA are seen predominantly. Although these observations have led some investigators to view these diseases as “ANCA-associated vasculitis,” the unique phenotypic features and absence of ANCA in some patients support that they should viewed as individual entities.
ANCA can also be seen in a broad range of nonvasculitic diseases where they are associated with antigens other than PR3 or MPO. Inflammatory bowel disease, autoimmune hepatitis, and cystic fibrosis can be associated with pANCA. In these diseases, ANCA react to a range of antigens that include bactericidal/permeability-increasing protein (BPI), lactoferrin, cathepsin G, lysozyme, and a myeloid cell–specific 50-kilodalton nuclear envelope protein. For this reason, a positive cANCA or pANCA should always be confirmed by target antigen specific testing for anti-PR3 and anti-MPO ANCA.
The initial observation that ANCA levels were higher in those with active disease raised hope that ANCA could be a biomarker for disease activity. In a prospective study of 156 patients, Finkielman et al demonstrated that PR3-ANCA levels were only weakly associated with disease activity, that decreases in PR3-ANCA levels during remission induction treatment were not associated with a shorter time to sustained remission, and that increases in PR3-ANCA levels were not associated with relapse in the following year.4 These results provide compelling evidence that serial ANCA levels should not be used to monitor disease activity or guide decisions about immunosuppressive treatment in WG.
The role of ANCA in disease pathogenesis remains unclear. Support for a pathogenic role for ANCA has come from in vitro data indicating potential mechanisms through which ANCA-mediated vascular injury could occur and from an elegant MPO knockout mouse model.5 These experiments demonstrated the induction of glomerulonephritis and vasculitis by the adoptive transfer of mouse-anti-MPO splenocytes into immune-deficient mice or the passive infusion of mouse anti-MPO immunoglobin G into both immune-deficient and immune-competent mice. However, the absence of ANCA in up to 20% of patients with WG and the ability for patients to have high ANCA levels yet remain in remission argue against ANCA being pathogenic.
Disease Activity
Assessment of disease activity forms the foundation for treatment decisions. In the absence of an effective biomarker, collective information from the history, physical examination, laboratories, and imaging remain the best means of detecting active disease. Although instruments such as the Birmingham Vasculitis Activity Score (BVAS) for WG are not generally used outside of studies, they highlight the manifestations of active disease in WG.6 It should always be kept in mind that many features, in particular pulmonary infiltrates or hematuria, are not specific for active WG, and the potential for chronic damage, infection, or medication toxicity must always be considered.
Treatment
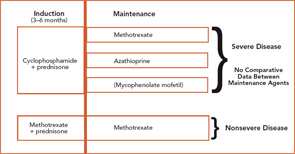
The current therapeutic approaches to WG are shown in Figure 1 (above).7 Treatment of WG is considered to have two phases: induction, where active disease is put into remission; and maintenance, where remission is sustained. CYC-sparing approaches have been developed to use this effective agent optimally for the treatment of severe disease while limiting CYC exposure to reduce the risk of toxicity.8
Induction
To date, the only agents proven to induce remission of active WG are prednisone in combination with either CYC or MTX. Patients with severe disease are usually treated with CYC 2 mg/kg/day and prednisone 1mg/kg/day. CYC should be given as a single dose in the morning with a large amount of fluid throughout the day to maintain a dilute urine. Because CYC is excreted by the kidney, dose reduction should be considered in patients with renal insufficiency. CYC treatment should be limited to three to six months in almost all instances, with subsequent transition to a maintenance agent.
The utility of daily oral versus intermittent intravenous (IV) CYC remains controversial, and the results from a randomized trial are not yet available. Although use of IV CYC has been based upon the rationale of side-effect reduction, it is not clear that the toxicity is less than daily CYC when given for three to six months combined with close monitoring.
Evidence from a randomized trial has supported that MTX can be used to induce remission of nonsevere WG.9 Oral or subcutaneous MTX is started at 15 mg/week (0.3 mg/kg/week) and increased within the first two to four weeks to 20–25 mg/week. To reduce toxicity, MTX is given with folic acid 1 mg daily or folinic acid 5 mg once a week taken 24 hours after MTX.
Maintenance
To date, there have been no comparative trials among individual maintenance agents. Therefore, a decision about which medication to use must be based on individual factors, including contraindications and relapse history. In the absence of cytopenias, the maintenance agent can be started within one to two days after stopping CYC.
For remission maintenance in WG, the largest body of data involves the use of AZA or MTX.10,11 AZA is given at a dose of 2 mg/kg/day, and MTX is given at doses as described for induction. Screening for thiopurine methyltransferase (TPMT) should be performed prior to AZA initiation when possible.
There has been a smaller body of published data with the use of mycophenolate mofetil (MMF)12 and leflunomide.13 MMF in particular may play a role in patients who cannot take AZA or MTX or those who have relapsed through these agents.
The optimal duration of maintenance therapy remains unclear. Although it is likely that relapse occurs more frequently in patients off treatment, prolonging therapy must be balanced against the potential for relapses that can still occur on medication, drug toxicity, and the ability of some patients to come off treatment. For newly diagnosed patients who do not have significant organ damage, treatment should be continued for at least two years in the absence of toxicity, after which time the clinician can consider tapering towards discontinuation. In patients who have relapsed or who have had severe permanent organ damage, treatment for a longer duration may become necessary.
Trimethoprim–Sulfamethoxazole
Because of its antimicrobial properties, the use of trimethoprim–sulfamethoxazole (T/S) for the treatment of WG has been difficult to assess. Use in isolated sinonasal disease can be considered with close observation, although it should never be used alone for other disease features. The role of T/S to reduce relapses was examined in a placebo-controlled randomized study and, although it lowered the rate of upper airways relapses, T/S did not prevent relapses in major organ sites.14
The most important role of T/S is in preventing Pneumocystis pneumonia, which can occur in 10% of WG patients receiving induction therapy. For patients on MTX, T/S has been safely given at prophylactic doses of trimethoprim 160 mg/sulfamethoxazole 800 mg three times a week or trimethoprim 80 mg/sulfamethoxazole 400 mg daily.
Fulminant Disease
Patients with pulmonary hemorrhage or rapidly progressive glomerulonephritis are often treated with IV methylprednisolone 1 g/day for three days together with CYC 3–4 mg/kg/day for three days, after which time doses are reduced to induction levels. Because there have been no data from standardized trials, this approach must weigh the unknown benefit against increased risk of infection.
In a recent study, 137 patients with rapidly progressive glomerulonephritis due to WG or MPA were treated with CYC and randomized to receive either plasma exchange (PE) or pulse methylprednisolone.15 PE increased the rate of renal recovery compared with methylprednisolone, although patient survival and the rate of severe adverse events were similar in both groups. This trial did not allow conclusions on the effectiveness of combining PE and methylprednisolone or on the impact of PE on other severe manifestations such as pulmonary hemorrhage.
Web Resource:
Patient Fact Sheet
To download the ACR’s patient fact sheet on WG, go to www.rheumatology.org and follow the links to patient education from the Practice Support Menu.
Biologic Therapies
Anti-TNF Therapy: The presence of granulomatous inflammation in WG raised interest in the use of anti–tumor necrosis factor (TNF) agents for the treatment of this disease. In the Wegener’s Granulomatosis Etanercept Trial (WGET), patients received standard induction-maintenance therapy and were randomized to receive etanercept or placebo.16 At the primary endpoint of sustained remission of over six months, there was no difference between etanercept and placebo, demonstrating that etanercept played no beneficial role in the treatment of WG. Investigators observed six malignancies in the etanercept arm; all malignancies had received concurrent CYC during the trial.17 Although this remains a focused experience, these data raise caution about the use of any anti-TNF therapy together with CYC. The divergent efficacy between etanercept, infliximab, and adalimumab in Crohn’s disease has raised the question as to whether other anti-TNF medications may be effective in WG. Until a sufficiently powered trial is performed, the available experience does not support the use of any anti-TNF agent in the routine care of patients with WG.
Rituximab: A promising body of data has emerged with the use of rituximab in WG. Following an initial case report, rituximab was studied in compassionate and open-label studies in combination with glucocorticoids.18,19 This experience found that rituximab was able to induce remission in association with B lymphocyte depletion and had a good safety profile. Although there have been a number of other small series, these data remain too small to draw conclusions regarding the efficacy of rituximab in WG. A randomized, double-blind, placebo-controlled trial is currently underway to compare rituximab to CYC for remission induction of severe active WG. Until data from this trial become available, rituximab should be considered investigational and should not be used in place of standard therapies.
Abatacept: The presence of activated T lymphocytes within WG lesions has raised the question as to whether interference with costimulation could modulate disease. The Vasculitis Clinical Research Consortium is currently conducting a pilot study investigating abatacept in mild relapsing WG. There has been no experience with abatacept in WG to date, and this should not be used in clinical practice.
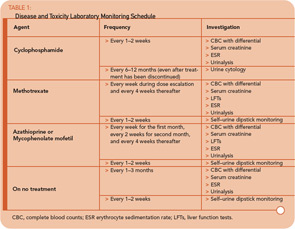
Monitoring and Toxicity Prevention
Monitoring for disease activity and medication toxicity is among the most important aspects of care in a patient with WG. The type and frequency of monitoring will depend largely on the medications the patient receives (see Table 1, p. 19). Monitoring blood counts to prevent neutropenia and maintain the absolute neutrophil count above 1500/mm3 lessens the risk of infection-related morbidity and mortality that can occur from bacterial and opportunistic pathogens. For patients who do not have blood in their urine, self–urine dipstick testing can help detect new hematuria as an early indicator of renal disease.
For patients with pulmonary disease, perform chest imaging one month after the initiation of induction treatment to confirm improvement, at the time of transition to maintenance therapy, and every three to six months thereafter. For patients who do not have pulmonary disease, perform imaging every six to 12 months or for new symptoms.
The Future
There is much we have left to learn about WG; understanding disease pathophysiology plays a critical role in future innovations. Gaining a greater knowledge of disease mechanisms will allow those in the field to make informed decisions when planning novel clinical trials.
In 2008, there is much that physicians can offer WG patients—this includes not only our current treatment and monitoring strategies but also hope for the many advances to come.
Dr. Langford is director of the Center for Vasculitis Care and Research and associate professor of medicine at Cleveland Clinic in Cleveland, Ohio.
References
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med. 1992;116:488–498.
- Merkel PA, Lo GH, Holbrook JT, et al. High incidence of venous thrombotic events among patients with Wegener granulomatosis: The Wegener’s Clinical Occurrence of Thrombosis Study. Ann Intern Med. 2005;142;620–626.
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet. 2006;368; 404–18.
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–619.
- Jennette JC, Xiao H, Falk RJ. Pahtogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2006;17:1235–1242.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides. Arthritis Rheum. 2001;44:912–920.
- Molloy ES, Langford CA. Advances in the treatment of small vessel vasculitis. Rheum Dis Clin North Am. 2006;32:157–172.
- Talar-Williams C, Hijazi YM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med. 1996;124: 477–484.
- De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–2469.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44.
- Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: Extended follow-up and rate of relapse. Am J Med. 2003;114:463–469.
- Langford CA, Tallar Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of Wegener’s granulomatosis. Arthritis Rheum. 2004;51: 278–283.
- Metzler C, Miehle N, Manger K, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford). 2007;46:1087–1091.
- Stegeman CA, Cohen Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. N Engl J Med. 1996;335:16–20.
- Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188.
- Wegener’s Granulomatosis Trial Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–361.
- Stone JH, Holbrook JT, Marriott MA, et al. Solid malignancies among patients in the Wegener’s Granulomatosis Etanercept Trial. Arthritis Rheum. 2006;54:1608–1618.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:262–268.
- Keogh KA, Ytterberg SR, et al. Rituximab for refractory Wegener’s granulomatosis: Report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180–187.