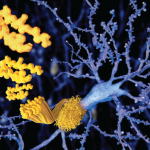
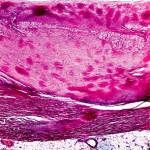
When stained with Congo red and observed under polarized light, amyloid deposits display an apple-green birefringence, a phenomenon of double refraction of light (see Figure 1). Abdominal fat pad aspiration has a positive predictive value of 98%, and a negative predictive value of 76% for diagnosing AL amyloidosis.5
For AL amyloidosis, serum and urine immunofixation electrophoresis will detect a monoclonal light chain in roughly 90% of patients.
If both screening biopsy and serum and urine immunofixation electrophoresis yield negative results, consider that the patient might have a hereditary form of amyloidosis (ATTR). This diagnosis can be confirmed either with isoelectric focusing to detect mutant transthyretin protein or restriction fragment length polymorphism (RFLP) analysis to detect a mutant gene.
If both techniques are negative, rarer forms of amyloidosis should be considered.

Imaging
Imaging modalities for amyloidosis include standard radiographs, MRI, ultrasound, and scintigraphic imaging.
Regarding standard radiography as it pertains to Aβ2M amyloidosis patients, Dr. Kay noted that, “in the axial skeleton, these patients may develop a destructive spondyloarthropathy which looks very much like septic diskitis.” Unless the radiologist is provided with a good history on the patient, or she or he likely will diagnose diskitis and recommend hospitalization with a course of antibiotics for your patient.
The precedent for using ultrasound imaging in AL amyloidosis was set in the late 1980s with echocardiography, used both to gauge disease impact on ejection fraction and to detect cardiac amyloid deposits. Prompted by this, Dr. Kay and colleagues developed criteria by which musculoskeletal ultrasound could be used to diagnose Aβ2M amyloidosis in dialysis patients.6
Scintography using radio-labeled amyloid P component, a ubiquitous constituent of all amyloid deposits, has been used to determine systemic involvement in amyloidosis, however, as Dr. Kay pointed out, this application is investigational, “and fraught with all the potential risks of using a biological product.”
Treatment
No medications are currently approved to treat patients with amyloidosis. Current strategies for disease management include supportive care, reduction of the amyloid precursor protein pool, stabilization of the native structure of precursor protein, and interference with constituents of amyloidosis fibrils.
In many cases, “one must rely on one’s internal medicine background to provide supportive care for these patients, such as when treating neuropathic pain or hypotension due to peripheral, or autonomic, neuropathy,” he said.
Preventative treatment in this setting is primarily directed at AL amyloidosis and, in general, employs regimens historically used to treat multiple myeloma. The goal of treatment is to reduce the amyloid precursor protein pool using:
- Melphalan + predisone ± colchicine
- Melphalan + high-dose dexamethasone
- Melphalan + dexamethasone + either thalidomide, lenalidomide, or bortezomib
- Clyclophosphamide + dexamethasone + lenalidomide
- Syngeneic or allogeneic bone marrow transplant
- Dose-intensive melphalan with autologous stem cell transplantation7
Commenting on this last approach, Dr. Kay said, “regardless of the potential and actual complications, dose-intensive melphalan with autologous stem cell transplantation offered a sea change in treating this devastating disease.” With treatment, estimated overall survival at five years is 47%.7 “That is a dramatic difference, as compared to the era before effective treatment was available,” he said.