Where were you Sept. 30, 2014?
By now you are all well aware of Section 6002 of the Affordable Care Act, which lays out the regulations of the Physician Payment Sunshine Act. Over a period of five months, August through December 2013, applicable manufacturers were tracking and reporting certain transfers of value made to U.S. physicians. As you may recall, you had the opportunity to review and dispute any erroneous reporting in August 2014. But where were you on Sept. 30, 2014? Were you online, scrutinizing the CMS Open Payments Database? If so, like many of your colleagues, you were likely disappointed by the lack of transparency in these government efforts to promote, well…transparency.
If you have visited the Open Payments site lately, you may have been equally as frustrated as the general public and media in navigating the site due to multiple unwieldy databases and significant limitations in search functionality. To further exasperate users, it’s not possible on the website to carry out queries, such as calculating the amount of money paid to institutions for a particular drug or comparing transfers of value from different companies. These deficiencies are just a few of the challenges of the system. CMS, however, has reassured all stakeholders that upgrades to this system are being made to enhance its overall performance.
In response to concerns about conveying potentially misleading information (at least in its current format), CMS felt compelled to remind the public that, “Just because there are financial ties doesn’t mean that anyone is doing anything wrong.” They stated, “Transparency will shed light on the nature and extent of these financial relationships and will hopefully discourage the development of inappropriate relationships.”
Although the intent is well-meaning, there are nuances to these data that may not be generally understood by those outside of healthcare.
Some Notes on the Data
Once the data were crunched by various stakeholders, it was determined that research payments from manufacturers to teaching hospitals and physicians equaled close to $1.5 billion. Although this total is an eye-catching number, part of this amount comes from the value of donated drugs intended for use by patients and does not represent direct monetary value to physician investigators. Will the general public and the media look only at the totals and derive their conclusions without drilling deeper into the data?
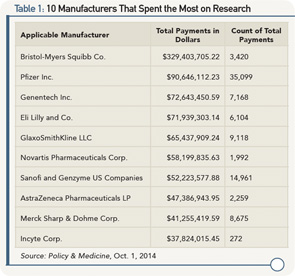
Lists have also been developed enumerating the top manufacturers by research spending over $5 million. The top 10 are listed in Table 1.
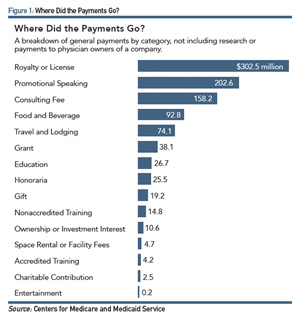
ProPublica, a nonprofit corporation based in New York that describes itself as an independent, nonprofit newsroom that produces investigative journalism in the public interest, analyzed CMS data from the various databases to track general payments from manufacturers. Most of the recipients of the highest transfers of value were in the surgical specialities (see Figure 1).
In the Meantime
While physicians were attempting to review and dispute reports on transfers of value from industry, the ACR, along with more than 100 other associations, including the AMA, was busy commenting on the proposed changes to the 2015 Sunshine Act and highlighting the importance of retaining the CME exemption. CMS noted that “industry support for accredited or certified continuing education is a unique relationship” and that accredited or certified continuing education payments to speakers are not reportable because they are not direct payments to a covered recipient.
Distressingly, the proposed changes had called for the deletion of the CME exemption, under which accredited CME providers, such as the ACR, could pay physicians for their participation as speakers/faculty in certified CME activities without sponsoring companies having to report the transfer of value to CMS. CMS had reasoned that removing the CME exclusion would avoid the redundancy in another section of the Final Rule that excluded indirect payments or other transfers of value where the applicable manufacturer is “unaware” of the identity of the covered recipient during the reporting year or by the end of the second quarter of the following reporting year. CMS had also named five accrediting bodies that were acceptable in meeting the CME exemption; removing the exemption would avoid any implied endorsement of specific accrediting bodies.
The ACR shared the viewpoint of most of the other medical specialty societies that the rule regarding indirect payments was ambiguous and insufficent to shield physicians from the requirement to report their involvement in CME. Moreover, the need of companies to be “unaware” of the recipients of funds for a period of 18 months was unreasonable given that CME activities are widely marketed and subject to media coverage.
A Modern Healthcare article quotes Andrew Rosenberg, senior advisor for The CME Coalition: “If it (the proposed CMS changes) is allowed to stand, this policy change will be massively disruptive to every stakeholder in the CME ecosystem—doctors, educators and commercial supporters—who have spent over a year preparing for the implementation of the current rules,” also adding that extending the disclosure to CME would “discourage physicians from learning new medical science by creating a false stigma.”
And the Verdict Is
Coincidence or not, on the night of Halloween, the CMS issued its changes to the Sunshine Act—not a trick, but more of a treat. In issuing these changes, the agency demonstrated that it had heard the concerns of the ACR and others by fixing the language of the policy in a way that effectively incorporated the CME exemption within other sections of the final rule. The CME community perceived this change as a huge victory. The regulation now states that as long as an applicable provider “does not require, instruct, direct, or otherwise cause the continuing education event provider to provide the payment to a covered recipient,” then it falls into the nonreportable category. CMS also put to rest concerns about the proposed time frame of “awareness” by applicable manufacturers of recipients of transfers of value. The revised policy states that payments remain unreportable even if the commercial supporter subsequently discovers the identity of the covered recipient.
Sun Shines on the ACR
Aside from CME, payments or transfers of value made to physicians indirectly through professional organizations, such as the ACR or the Rheumatology Research Foundation, are reportable if the manufacturer knows that money will be paid to physicians or earmarks the money to go to physicians. Although the ACR and/or the Foundation do not make any reports to the government as a result of the Sunshine Act, it will be required to report back to those companies from which they receive financial support. To this end, the ACR will be collecting physicians’ NPI numbers through the online ACR My Profile and during registration of its various activities.
It’s important to emphasize that speakers at CME-accredited meetings or attendees at these meetings are not reportable to the sponsoring company. However, some activities are reportable, such as accepting payment for speaking at a non-CME event sponsored by the ACR, receipt of certain grants from the Foundation or attending a company-sponsored event.
The ACR and the Foundation will tell its members ahead of time whenever an ACR or Foundation activity is reportable to a company sponsor. In this way, members will have the opportunity to decline participating in an activity if they wish to avoid having this information being reported back to a company.
The Sunshine Act continues to be a hot topic at the ACR. The Board of Directors was informed about the proposed changes to this policy at its August meeting when it was discussed by Joel Block, MD, chair of the Committee on Education. Members have been kept apprised of the reporting and dispute process through articles in RheumWatch and the Rheumatology Morning Wire. In light of these evolving policies, task forces have been formed by both the ACR and the Foundation that will address the ongoing challenges posed by the Sunshine Act and will work in parallel to develop policies, procedures, and action plans that will ensure compliance with these regulations while retaining positive and fruitful relationships with industry.
In the areas of advocacy, government affairs, and communications, the ACR will continue to bring you the latest information about the Sunshine Act and what it means to you, as well as keep you up to date about our formal positions on this issue. It’s critical for the ACR to continue its exemplary practices as an accredited CME provider and, where necessary, comply with the requirements for timely and accurate reporting. By taking this approach, the ACR will ensure you receive the highest quality education and stay true to its mission of Advancing Rheumatology!
Extending the disclosure to CME would ‘discourage physicians from learning new medical science by creating a false stigma.’
What You Can Do
As noted in the piece by Herb Barafs, MD, in the Nov. 5 issue of RheumWatch: Look at your data now, if you haven’t already done so. Know what’s being reported about “payments” to you. Begin disputing data where it’s necessary. Here’s how:
- Complete the CMS e-verification process through its Enterprise Portal (https://portal.cms.gov). This is a two-phase registration process. CMS has outlined instructions for physicians (http://www.cms.gov/Regulations-and-Guidance/Legislation/National-Physician-Payment-Transparency-Program/Downloads/Phys-Teaching-Hosp-Phase-1-EIDM-Registration-Steps-%5bJune-2014%5d.pdf).
- Register with the CMS Open Payments System (https://portal.cms.gov/wps/portal/unauthportal/registration). You must first be e-verified through the Enterprise Portal.
- Review data and dispute inaccuracies. CMS produced quick reference guides to assist with getting into the system and reviewing and disputing data, found at http://www.cms.gov/Regulations-and-Guidance/Legislation/National-Physician-Payment-Transparency-Program/Physicians.html.
- Check the Sunshine Act toolkit (http://www.ama-assn.org/ama/pub/advocacy/topics/sunshine-act-and-physician-financial-transparency-reports/sunshine-act-toolkit.page). The AMA has put together this resource for physicians.

E. William St.Clair, MD, is president of the ACR and chief of the Duke Division of Rheumatology and Immunology. Dr. St.Clair, a rheumatologist, has 25 years of experience as a clinical investigator. Contact him at [email protected].