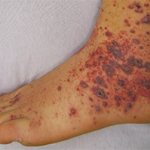
EULAR 2024 (VIENNA)—How should we treat immunoglobulin A (IgA) vasculitis and IgA nephropathy? For patients requiring immunosuppression, glucocorticoids remain a first-line treatment, and it remains unclear what glucocorticoid-sparing agents work best.
EULAR 2024 (VIENNA)—How should we treat immunoglobulin A (IgA) vasculitis and IgA nephropathy? For patients requiring immunosuppression, glucocorticoids remain a first-line treatment, and it remains unclear what glucocorticoid-sparing agents work best.
To date, no clinical practice guidelines exist to inform this decision making in an evidence-based manner. The good news: That will soon change. Consensus guidelines are being developed by the European IgA vasculitis Study Group (EUGAVAS-initiative) and should be complete within the next few years.
In a session at EULAR 2024, Jens Thiel, MD, professor of medicine and director of the Division of Rheumatology and Clinical Immunology, Medical University of Graz, Austria, provided an update on the treatment of IgA vasculitis and IgA nephrology.
IgA Refresher
IgA vasculitis is relatively rare, affecting only about 1.5–51 in 1 million adults. The disease affects twice as many men as women, with onset typically between ages 40 and 60. Unfortunately, relapsing courses are common.1
Clinically, IgA vasculitis may involve the skin, joints, kidneys and/or intestines. All patients experience skin involvement, which manifests as palpable purpura predominantly on the lower extremities (e.g., necrotic lesions, urticarial lesions or pustules). About one-third of patients have constitutional symptoms, and 60–80% have non-destructive arthralgias/arthritis of the knees, wrists, ankles or small joints.2 Renal involvement affects 70% of patients and often has a delayed onset. It may manifest as asymptomatic hematuria, nephrotic syndrome or even terminal renal insufficiency. Gastrointestinal manifestations can include abdominal pain, nausea/vomiting, bleeding, ischemia and perforation.3 Abdominal pain in IgA vasculitis is caused by submucosal hemorrhage and edema.
Treatment
Currently, the treatment of IgA vasculitis varies on the basis of clinical manifestations and regional approaches to care. This situation is not surprising due to the heterogeneity of clinical presentation, lack of treatment guidelines and few randomized controlled trials to guide practice. Treatment options include such agents as topical glucocorticoids, systemic glucocorticoids, colchicine, dapsone, azathioprine, rituximab and cyclophosphamide. This list may feel overwhelming, especially when limited evidence is available to guide drug selection.
Treatment Data
Dr. Thiel began by reviewing the existing data for current treatments for IgA vasculitis and IgA nephropathy.
- Cyclophosphamide: In 2010, a small randomized controlled trial, the CESAR study, compared glucocorticoids monotherapy with glucocorticoids plus cyclophosphamide.
Dr. Thiel said, “There was no difference in the primary end point of complete disease remission, but there seemed to be a survival advantage for combination therapy.”4
- Rituximab: In 2020, a systematic review showed that rituximab led to some clinical improvement in about 95% of patients with IgA vasculitis and sustained disease remission at the end of follow-up in about 74% of patients. However, relapse was relatively common, at almost 40%.
“As for IgA nephropathy, results were disappointing with no difference in estimated glomerular filtration rate [eGFR] or proteinuria. We need more data,” Dr. Thiel said.5
- Dapagliflozin: Dapagliflozin is a sodium-glucose cotransporter-2 (SGLT2) inhibitor that was studied in patients with IgA nephropathy in 2021.
“Patients taking dapagliflozin did much better compared with [placebo-treated patients] in regard to eGFR decline, end-stage kidney disease, and renal and cardiovascular death,” said Dr. Thiel.6
- Targeted-release budesonide: This treatment is a novel, oral, targeted-release formulation of budesonide designed to act at the gut mucosal level. A phase 3 randomized controlled trial showed that targeted-release budesonide resulted in a significant decrease in proteinuria, as well as preservation of eGFR, when compared with placebo in patients with IgA nephropathy.7,8
“This [treatment approach] may become of interest,” said Dr. Thiel.
Future Possibilities
Dr. Thiel concluded with a discussion of therapeutics coming down the pipeline.
- Sibeprenlimab: Sibeprenlimab binds and neutralizes a proliferation-inducing ligand (APRIL), which regulates B-cell mediated immune responses. In November 2023, phase 2 data showed a significant decrease in proteinuria and slowing of eGFR decline in patients with IgA nephropathy.9
“This [agent] may really be an interesting target in IgA vasculitis, and a phase 3 trial is ongoing in IgA nephropathy,” said Dr. Thiel.
Of note, belimumab and blisibimod, which inhibit B cell activating factor (BAFF), won’t be further investigated for this condition.
- Atacicept: Atacicept is a dual inhibitor of BAFF and APRIL. In March 2024, phase 2b data showed decreases in proteinuria and stabilization of eGFR in patients with IgA nephropathy treated with atacicept. “Results look really encouraging,” Dr. Thiel said.10
- Complement-targeting therapies (e.g., avacopan): “Most of these [treatments] are in early phase studies,” said Dr. Thiel. “Some results have been positive. Some trials have been halted. It’s not yet clear, in my opinion, whether they’ll really be a success.”
- Sparsentan: In a phase 3 trial of patients with IgA nephropathy, sparsentan, a novel, non-immunosuppressive, dual endothelin angiotensin receptor antagonist, was compared with irbesartan, an angiotensin II receptor blocker. Like several of the therapies mentioned above, sparsentan showed a significant better in proteinuria and stabilization of eGFR in this patient population.11
Conclusion
Currently, the evidence remains unclear for the best treatment for adults with IgA vasculitis. The clinical spectrum of IgA vasculitis is broad, and the therapeutic spectrum ranges from symptomatic treatment to cytotoxic therapies.
“Glucocorticoid therapy remains a first-line [treatment], if systemic immunosuppression is needed,” said Dr. Thiel. “Targeted-release budesonide may be of interest in IgA-V, and there are many other drugs under investigation. The EUGAVAS IgA vasculitis guidelines are eagerly awaited.”
Samantha C. Shapiro, MD, is the executive editor of Harrison’s Principles of Internal Medicine. As a clinician educator, she practices telerheumatology and writes for both medical and lay audiences.
References
- Thalen M, Gisslander K, Segelmark M, et al. Epidemiology and clinical characteristics of biopsy-confirmed adult-onset IgA vasculitis in southern Sweden. RMD Open. 2024;10(1):e003822.
- Neumann T. Update on immunoglobulin A vasculitis. Z Rheumatol. 2022;81(4):305–312.
- Audemard-Verger A, Pillebout E, Amoura Z, et al. Gastrointestinal involvement in adult IgA vasculitis (Henoch-Schönlein purpura): Updated picture from a French multicentre and retrospective series of 260 cases. Rheumatology (United Kingdom). 2020;59(10):3050–3057.
- Pillebout E, Alberti C, Guillevin L, et al. Addition of cyclophosphamide to steroids provides no benefit compared with steroids alone in treating adult patients with severe Henoch Schönlein Purpura. Kidney Int. 2010;78(5):495–502.
- Hernández-Rodríguez J, Carbonell C, Mirón-Canelo JA, et al. Rituximab treatment for IgA vasculitis: A systematic review. Autoimmun Rev. 2020;19(4):102490.
- Wheeler DC, Toto RD, Stefánsson B V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–224.
- Barratt J, Lafayette R, Kristensen J, et al. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023;103(2):391–402.
- Lafayette R, Kristensen J, Stone A, et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. The Lancet. 2023 Sep 9;402(10405):859–870.
- Mathur M, Barratt J, Chacko B, et al. A phase 2 trial of sibeprenlimab in patients with IgA nephropathy. N Engl J Med. 2024 Jan 4;390(1):20–31.
- Lafayette R, Maes B, Lin C, et al. #3848 origin trial: 24-wk primary analysis of a randomized, double-blind, placebo-controlled ph2B study of atacicept in patients with IgAN. Nephrol Dial Transplant. 2023 Jun;38(suppl 1).
- Rovin BH, Barratt J, Heerspink HJL, et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet. 2023 Dec 2;402(10417):2077–2090.