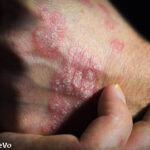
(Reuters)—Drug developer XenoPort Inc. said on Tuesday its experimental drug was effective in treating psoriasis, sending its shares up 19% in premarket trading.
The oral drug met the main goal in a phase 2 trial of patients with moderate-to-severe chronic plaque-type psoriasis, the company said.
XenoPort said it expected to start late-stage trials next year and that it would explore partnerships to speed up the development of the drug globally.
According to the National Institutes of Health, psoriasis is estimated to affect 2.0-2.6% of the U.S. population, with higher incidence in Caucasians. About 15% of psoriasis patients may eventually develop psoriatic arthritis.
The drug, now called XP23829, “met its primary endpoint in both 800 mg once daily and 400 mg twice daily doses, demonstrating statistically significant improvements in percent change from baseline to week 12 in Psoriasis Area and Severity Index (PASI) score,” the company said in a news release. “XP23829 was safe and generally well tolerated.”
“XP23829 is a patented prodrug of monomethyl fumarate (MMF) in a novel oral formulation that was designed to potentially offer physicians and patients an effective, better tolerated and easier to use therapeutic option to currently available fumarate products,” XenoPort said.
The U.S. Food and Drug Administration earlier this year approved Novartis’ injectable drug Cosentyx (secukinumab) for psoriasis. Eli Lilly & Co is also developing Ixekizumab for treating the disease.
Canada’s Valeant Pharmaceuticals International Inc. also bought rights to AstraZeneca Plc’s late-stage experimental psoriasis drug brodalumab after it was dropped by Amgen Inc in May.