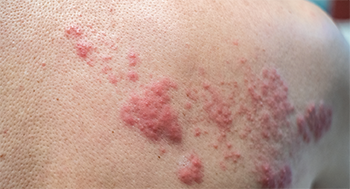
This rash shows the effects of varicella-zoster reactivation—shingles.
Mumemories / shutterstock.com
Varicella-zoster-virus (VZV) reactivation, which can cause patients to develop herpes zoster (i.e., shingles), occurs more frequently in patients with systemic vasculitis and systemic lupus erythematosus (SLE) who have received intravenous cyclophosphamide than in otherwise healthy adults, according to a retrospective study published in The Journal of Rheumatology by researchers in France.1 The study also shows that antiviral chemoprophylaxis with valacyclovir is an effective protective option for these patients.
“Zoster is an important burden for these patients, as described in our paper and papers from other groups. Moreover, when it occurs, it makes the choice of lowering the immunosuppressant treatment—or not—more difficult if the underlying disease is not under control,” says Guillaume Moulis, MD, PhD, assistant professor in internal medicine at the University of Toulouse, and one of the study’s co-authors.
Rheumatologists often prescribe intravenous cyclophosphamide to treat severe SLE flares and systemic vasculitis, along with corticosteroids. Cyclophosphamide exposure increases the risk of severe infections.2-4
Exploring Zoster Risks
The study’s goals were to assess zoster incidence and risk factors in patients with SLE or systemic vasculitis who have been exposed to intravenous cyclophosphamide.
Although evidence shows SLE patients are at higher risk for zoster, there had been little data on the risk among patients with systemic vasculitis.5 Past studies show that zoster is a concern for patients with granulomatosis with polyangiitis (GPA) and that treatment with corticosteroids and cyclophosphamide is a possible factor.6,7 In GPA, kidney failure may be one reason for this higher incidence of zoster.8
Because immunocompromised patients and those on moderate to high doses of immunosuppressive therapy, including cyclophosphamide, were excluded from clinical efficacy studies for the new recombinant zoster vaccine, Shingrix, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) does not currently recommend its use in these patients, although the committee will likely review these recommendations as more data become available.9 One of the aims of this study was to assess whether valacyclovir could be an effective, protective option for these patients.
“This study started from the experience of zoster flares in our patients, with a lack of data to explain the evolution of this to them, and from the observation that some patients had a chemoprophylaxis by valacyclovir, and others not,” says Dr. Moulis. “Consequently, we conducted this study to extend the knowledge about zoster in this setting, and to try to demonstrate the potential effectiveness of valacyclovir in this setting as well. These results impacted our practice.”
Since the study’s completion, physicians at his hospital now systematically prescribe valacyclovir for unvaccinated patients with the identified risk factors, says Dr. Moulis.
Study Details
This retrospective study included adults treated with intravenous cyclophosphamide for either SLE or systemic vasculitis between 2011 and 2015 at Toulouse University Hospital. They recorded zoster occurrence based on medical charts, laboratory data and patient interviews. They assessed zoster risk factors and the protective effect of valacyclovir prophylaxis using univariate Cox models.
The study cohort included 110 patients: 81 with systemic vasculitis and 29 with SLE. Patients in the cohort were 18 years of age or older with a median age of 50 years old. Their mean age at time of first exposure to cyclophosphamide was 55.7 years old. During a mean follow-up period of 3.4 years after the patients initiated cyclophosphamide, there were 10 cases of zoster—an overall incidence of 27.9/1,000 patient-years. The incidence was 59.4/1,000 patient-years during the first year after cyclophosphamide initiation. Four of the patients who developed zoster had persistent postherpetic neuralgia.
The study showed that both lymphopenia <500/µl at cyclophosphamide initiation and female sex are probably zoster risk factors. Patients with SLE had a higher incidence of zoster than those with systemic vasculitis.
Nineteen patients in the cohort, or 17.2%, were treated with 500 mg of oral valacyclovir either once or twice a day during the follow-up period. None of these patients developed zoster during the follow-up. Two of these patients had a history of zoster, five had SLE, and 16 started valacyclovir and cyclophosphamide at the same time. Mean duration of corticosteroid exposure in these 19 patients was 3.6 years, and the mean cumulative cyclophosphamide dose was 5,914 mg. The co-authors noted that 20.5% of the patients exposed to a high cumulative dose of cyclophosphamide, or greater than 3,000 mg, were exposed to valacyclovir prophylaxis, compared to 10.3% of patients who were exposed to a lower cumulative dose of cyclophosphamide. No adverse reactions to valacyclovir were observed during this study.
The co-authors concluded that zoster incidence is high in both systemic vasculitis and in SLE patients exposed to intravenous cyclophosphamide, and that cyclophosphamide may increase a patient’s risk of postherpetic neuralgia. They encourage rheumatologists managing SLE and systemic vasculitis patients to prescribe prophylaxis with valacyclovir, especially in patients with lymphopenia <500/µl at cyclophosphamide initiation and during the following year.
Higher Severity
In this cohort, 30% of the patients with zoster had to be hospitalized for management, and 40% developed postherpetic neuralgia. “We were neither surprised nor not surprised by this finding. We just didn’t know,” he says. The rates for both were high compared with previous cohorts of immunocompromised patients, and the co-authors suspect that cyclophosphamide exposure may be a risk factor for the lingering neuralgia.
None of the patients in this cohort had been vaccinated against VZV. Although the inactivated zoster vaccine may be effective protection for people with autoimmune diseases, the co-authors stress that SLE and systemic vasculitis patients generally receive intravenous cyclophosphamide as an urgent treatment for a severe flare. They may not have time to be vaccinated before immunosuppressant treatment is started, so valacyclovir is an effective option, they concluded.
Risks of valacyclovir prophylaxis include “the well-known adverse drug reactions,” including fatigue, nausea, vomiting, stomach pain, dizziness, sore throat, skin rash, headache, depression and stuffy nose, “although none were observed our study,” says Dr. Moulis.
Rheumatologists should be more aware of the potential risk of zoster reactivation from cyclophosphamide exposure in patients with SLE and systemic vasculitis, and consider valacyclovir prophylaxis in cases where patients have not already been vaccinated against VZV, Dr. Moulis says. Prospective studies are needed to confirm these results, he adds.
Susan Bernstein is a freelance medical journalist based in Atlanta.
References
- Garnier C, Ribes D, Chauveau D, et al. Zoster after cyclophosphamide for systemic lupus erythematosus or vasculitis: Incidence, risk factors, and effect of antiviral prophylaxis. J Rheumatol. 2018 Jul;180310. Epub ahead of print.
- Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008 Feb;67(2):195–205.
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016 Sep;75(9):1583–1594.
- Hoffman GS, Leavitt RY, Fauci AS, et al. Infectious complications of cyclophosphamide treatment for vasculitis. Arthritis Rheum. 1989 Dec;32(12):1626–1627.
- Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014 Apr;42(2):325–334.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: An analysis of 158 patients. Ann Intern Med. 1992 Mar 15;116(6):488–498.
- Charlier C, Henegar C, Launay O, et al. Risk factors for major infections in Wegener granulomatosis: Analysis of 113 patients. Ann Rheum Dis. 2009 May;68(5):658–663.
- Wung PK, Holbrook JT, Hoffman GS, et al. Herpes zoster in immunocompromised patients: incidence, timing, and risk factors. Am J Med. 2005 Dec;118(12):1416.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018 Jan 26;67(3):103–108.
