First described in 1888, polymyalgia rheumatica (PMR) is a chronic inflammatory condition that almost exclusively affects individuals older than 50.1 Until this week, no therapies for PMR had been approved by the U.S. Food & Drug Administration (FDA).2 Tuesday, Sanofi announced sarilumab (Kevzara) has been approved by the U.S. Food & Drug Administration for the…
Cardiovascular Safety with RA Treatments
Research by Chicre et al. found that Janus kinase inhibitors may significantly increase the risk of major adverse cardiac events and all-cause death in patients with rheumatoid arthritis (RA) when compared with other RA treatments. This study highlights the need for more comparative safety studies.

Supplemental Vitamin D May Not Protect Against Fractures in Healthy Adults
Supplemental vitamin D may not significantly lower the risk of fractures in generally healthy adults compared with placebo, according to a large study by LeBoff et al.

Point-of-Care Uric Acid Testing
In June 2022, I listened to several presentations on gout at EULAR’s European Congress of Rheumatology. Most began with data confirming a sad truth that we, as rheumatology providers, are all aware of: too many patients are taking subtherapeutic doses of urate-lowering therapy (ULT).1,2 Recommendations from the American College of Physicians in 2017 advocated for…
2022 ACR Guideline for Vaccinations in Patients with RMDs
This guideline provides evidence-based recommendations on the use of vaccinations in children and adults with rheumatic and musculoskeletal diseases. It includes expanded indications for some vaccines, as well as guidance on whether to hold immunosuppressive medications or delay vaccination to maximize vaccine immunogenicity and efficacy.
Glucocorticoids May Decrease White Matter Integrity & Change Gray Matter Volume
Van der Meulen et al. found the use of both systemic and inhaled glucocorticoids is associated with changes in several brain imaging parameters, including decreased white matter integrity and gray matter volume. Study patients also reported more depressive symptoms and tiredness than controls.

FDA Approves Abaloparatide to Treat Men with Osteoporosis & a High Risk of Fracture
In late December, the FDA approved subcutaneous abaloparatide for the treatment of men with osteoporosis at a high risk of fracture. This approval is based on a placebo-controlled study that showed abaloparatide led to significant increases in bone mineral density of the lumbar spine, total hip and femoral neck. Abaloparatide was approved in April 2017 for the treatment of postmenopausal women with osteoporosis at high risk for fracture.

ACR Convergence 2022 Closing Session Discusses Research Highlights
PHILADELPHIA—Expert panelists gathered in the closing session at ACR Convergence 2022 to give their take on what they saw as some of the most notable research findings and other insights to come out of the meeting, touching on a number of topics on the leading edge of the field. COVID-19 Prophylaxis & Vaccinations Alfred Kim,…

Top 10 Tricks for the Management of Dry Mouth
PHILADELPHIA—Whether due to Sjögren’s disease or something else, dry mouth is a common chief complaint from patients with rheumatic illnesses. Dry mouth isn’t life-threatening, but it can have a serious impact on quality of life. Sialogogues like cevimeline and pilocarpine may benefit some, but not all, patients, but cholinergic side effects often limit their usefulness….
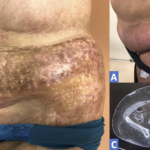
ACR Image Competition 2022 Results, Part 3
Localized Scleroderma in Anti-NXP2-Antibody Positive Dermatomyositis A 67-year-old woman presented with erythematous, indurated skin on her left flanks. She had been diagnosed with dermatomyositis one year earlier when proximal muscle weakness, dysphagia and skin rash developed (see Figure A). Tests at the time showed the presence of anti-NXP2 and anti-Ro52 antibodies, as well as pathological…
- « Previous Page
- 1
- …
- 31
- 32
- 33
- 34
- 35
- …
- 337
- Next Page »