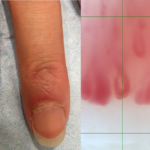
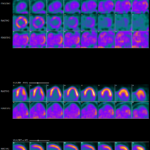
Periungual Erythema & Its Translation on Nailfold Videocapillaroscopy in a Patient with Very Early Systemic Sclerosis A 66-year-old woman presented with Raynaud’s phenomenon and periungual erythema. HEp-2 immunofluorescence assay was positive for antinuclear antibodies, showing a centromere pattern. The presence of anti-centromere antibodies was confirmed by chemiluminescent immunoassay. The patient was diagnosed with very early…