Dr. Jacob van Laar took a deep dive into the what defines difficult-to-treat rheumatoid arthritis & how to approach these patients during EULAR 2022.
Subcategories:Axial SpondyloarthritisClinical Criteria/GuidelinesGout and Crystalline ArthritisMyositisOsteoarthritis and Bone DisordersOther Rheumatic ConditionsPain SyndromesPediatric ConditionsPsoriatic ArthritisRheumatoid ArthritisSjögren’s DiseaseSoft Tissue PainSystemic Lupus ErythematosusSystemic SclerosisVasculitis

Dr. Jacob van Laar took a deep dive into the what defines difficult-to-treat rheumatoid arthritis & how to approach these patients during EULAR 2022.

This EULAR 2022 session emphasized the importance of recognizing the axial manifestations of psoriatic arthritis and treating these symptoms accordingly.

A study from Haibel et al. in patients with chronic knee arthritis found intra-articular morphine did not lead to a significant, short-term reduction in pain compared with placebo and proved inferior to treatment with intra-articular triamcinolone.

EULAR 2022 (VIRTUAL)—The pace of scientific progress in research medicine is incredible and seems to only accelerate with time. Thus, the 2022 Congress of the European Alliance of Associations for Rheumatology (EULAR) session on late-breaking abstracts fittingly captured the excitement and timeliness of a number of research projects that have just recently been completed and…

In this EULAR 2022 session, new & revised treatment recommendations for ANCA-associated vasculitis, axial spondyloarthritis & rheumatoid arthritis were presented.
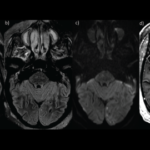
Fazila Aseem, MD, MPH, Alexander D. Jeffs, MD, Enid Y. Sun, MD, MPH, Randaline R. Barnett, MD, Courtney Blodgett, AG-ACNP, Winnie Lau, MD, Casey Olm-Shipman, MD, MS, Matthew F. Sharrock, MD, Rhonda Cadena, MD, Yueh. Z. Lee, MD, PhD, Alfredo C. Rivadeneira, MD, & Clio A. Rubinos, MD, MS |
Scleromyxedema is a primary cutaneous mucinosis characterized by a diffuse and generalized papular skin eruption of mucinous deposits throughout the upper dermis. In addition to dermatologic manifestations, scleromyxedema may involve the cardiopulmonary, gastrointestinal, renal and nervous systems. Dermato-neuro syndrome (DNS) is a rare, severe neurologic complication of scleromyxedema.1,2 The pathogenesis of DNS is unknown, but…
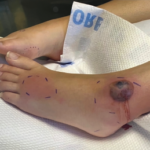
Ryan Guerrettaz, MD, Angelo Ciliberti, MD, Rochella Ostrowski, MD, Elise Wolff, DO, Nadia Qureshi, MD, & Ramzan Shahid, MD |
Acute febrile neutrophilic dermatosis, or Sweet syndrome, is an inflammatory disease that classically presents with fever, leukocytosis and tender, erythematous plaques characterized by neutrophilic infiltrates on biopsy. Sweet syndrome has been reported in association with several autoimmune diseases, including inflammatory bowel disease, systemic lupus erythematous, rheumatoid arthritis and sarcoidosis.1 Here, we discuss a case of…
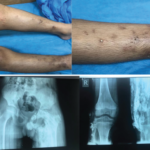
Hala Fadhil Hasan, MBChB; Featured Image from Middle East & North Africa |
Rheumatic Diseases of Childhood: Juvenile Dermatomyositis with Calcinosis Cutis These images depict a 14-year-old boy with a two-year history of proximal muscle weakness affecting both upper and lower limbs, and a skin rash affecting his face. He was diagnosed with juvenile dermatomyositis and developed calcinosis over both legs with skin infection and ulceration. Plain X-ray…

Joseph Cantrell, JD, & Kenneth G. Saag, MD, MSc |
The ACR and a new Access to Reproductive Health Care Task Force are working to ensure patients with rheumatic disease—particularly women—have access to the medications and treatments they need, including methotrexate, and that rheumatology providers are able to maintain trusting relationships with and advise their patients on all matters relevant to the management of their rheumatic diseases.

Keri Losavio & Julie Nusbaum, MD |
In light of new challenges to individuals’ reproductive rights and the known challenges of clinical management of rheumatic disease patients during pregnancy, we review the current state of reproductive rheumatology and the management of patients with rheumatic disease during pregnancy.