Yalamanchili et al. describe how trends in disease-modifying anti-rheumatic drug (DMARD) use have evolved for insured, U.S. patients with juvenile idiopathic arthritis. Overall, the study found that from 2000 to 2022 in this patient population the use of biologic and targeted synthetic DMARDs rose, while the use of conventional synthetic DMARDs declined.
ACR Image Competition 2024 Results, Part 4
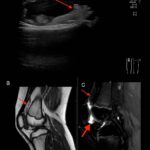
For the 2024 Image Competition, the ACR sought images with educational or remarkable manifestations representing a diverse range of pediatric patients with autoimmune, inflammatory, infectious and malignant drivers of rheumatic disease. Here, we showcase the winning images from the Middle East and North Africa. Lipoma Arborescens Revealed A 10-year-old boy, presented with a five-year history…

Youthful Exuberance: The Year in Review for Pediatric Rheumatology
WASHINGTON, D.C.—It is no small task to summarize an entire year’s worth of research accomplishments in any field of medicine, let alone one as complex as rheumatology. At ACR Convergence 2024, the Pediatric Year in Review not only provided a thoughtful summary, but also grouped advances along several different themes. Immune Health & More Jessica…

Weathering the Cytokine Storm: Macrophage Activation Syndrome in Adults & Children
Peter Nigrovic, MD, provides a practical guide to the diagnosis & treatment of macrophage activation syndrome (MAS) in children & adults.

FDA Officials Discuss Extrapolation & Confusion Around Pediatric Therapies
A session at ACR Convergence 2024 addressed several recent drug approvals in pediatric rheumatology and a safety update for IL-1/IL-6 inhibitors.

Can Cultural Humility Conquer Systemic Inequity in Pediatric Rheumatology?
Doctors and patient advocates urged the rheumatology community to address the drastic inadequacies in care faced by marginalized people in a session held at ACR Convergence 2024.

The Great Pediatric Debate 2024: Cessation or Continuation of Biologics in sJIA Lung Disease?
Washington D.C.—At a Sunday, Nov. 17, Pediatrics Great Debate session of ACR Convergence, speakers argued whether patients with systemic juvenile idiopathic arthritis (sJIA) should continue their interleukin (IL) 1/IL-6 biologics if lung disease is suspected. Randy Q. Cron, MD, PhD, the director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham,…

SURF’n Autoinflammation: Evaluation & Management of Syndrome of Undifferentiated Recurrent Fever
At this ACR Convergence 2024 session, the focus was on the identification & treatment of a newly identified syndrome of undifferentiated recurrent fever (SURF).
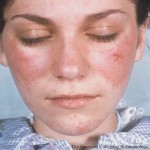
Personalize Your Pediatric Lupus Care
On Nov. 16, the ACR Convergence 2024 session Toward Personalized Medicine in Pediatric Lupus will present important recent findings in the pathophysiology of pediatric lupus. Virginia Pascual, MD, is program director of an NIAID-funded Autoimmunity Center of Excellence and a NIAMS-funded Center for Lupus Research at Weill Cornell Medical College, New York. Her laboratory is…

Spotlight on Pediatric Rheumatology in Singapore
A pioneer of pediatric rheumatology in Singapore, Dr. Elizabeth Ang details her unique clinical experiences.
- 1
- 2
- 3
- …
- 13
- Next Page »