Yalamanchili et al. describe how trends in disease-modifying anti-rheumatic drug (DMARD) use have evolved for insured, U.S. patients with juvenile idiopathic arthritis. Overall, the study found that from 2000 to 2022 in this patient population the use of biologic and targeted synthetic DMARDs rose, while the use of conventional synthetic DMARDs declined.
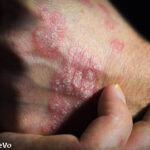
Deucravacitinib Promising for PsA
Results from two studies found that deucravacitinib improved the signs and symptoms of patients with psoriatic arthritis who were biologic disease-modifying anti-rheumatic drug naive and those previously treated with a tumor necrosis factor α inhibitor.

FDA Approval Sought for 2 Pediatric Indications for Guselkumab
The FDA is reviewing supplemental biologics license applications for guselkumab to treat children with juvenile psoriatic arthritis and moderate to severe plaque psoriasis.
mRNA CAR T Cell Therapy Receives FDA’s Rare Pediatric Designation to Treat Juvenile Dermatomyositis
FDA has granted Descartes-08, an mRNA chimeric antigen receptor T cell therapy, a rare pediatric disease designation for the treatment of juvenile dermatomyositis.

Reproductive Health, Biosimilars & More in Focus at SOTA 2025
Reproductive health, biosimilars, IgG4-related disease and much more—five speakers give us a sneak peek into important topics being addressed at the ACR’s 2025 State-of-the-Art Clinical Symposium, April 4–6.

FDA Approves Bimekizumab-bkzx (Bimzelx) for 3 New Rheumatic Indications
The FDA has approved bimekizumab-bkzx for the treatment of adults with psoriatic arthritis, non-radiographic axial spondyloarthritis and ankylosing spondylitis.

The Ethical Tug-of-War Over Biosimilar Adoption
The advent of biosimilar medications has offered the promise of significant cost savings for healthcare systems and patients. Biosimilars are highly similar versions of existing biologic drugs, providing a more affordable alternative once the original biologic patent expires. However, the adoption of biosimilars in the U.S. has been hampered by myriad roadblocks, many of which…

AC&R Study Summary: Standardizing Treatment for Moderately Severe JDM
Why was this study done? Juvenile dermatomyositis (JDM) is the most common type of idiopathic inflammatory myopathy in childhood, and most patients have a chronic disease course requiring prolonged administration of systemic glucocorticoids and immunosuppressive agents. The initial management for patients with moderately severe JDM is relatively standardized, typically including methotrexate and systemic glucocorticoids with…

Sonelokimab Promising for Patients with PsA
A phase 2 clinical trial has demonstrated the effectiveness for multiple doses of subcutaneous sonelokimab for the treatment of adults with active psoriatic arthritis.

15,000 Patient-Years: Safety Data for Upadacitinib Treatment Across Multiple Rheumatic Conditions
Burmester et al. characterized the long-term safety profile of upadacitinib treatment across multiple rheumatic diseases.
- 1
- 2
- 3
- …
- 40
- Next Page »