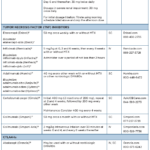
About 30% of patients with psoriasis have psoriatic arthritis (PsA), a complex, multi-faceted, chronic, inflammatory musculoskeletal and skin disease for which the treatment has changed considerably over the past few years.1 Biosimilars and other new drugs have become a therapeutic turning point for many patients suffering from rheumatic illnesses, including PsA. The treatment of PsA…