According to a German study presented during ACR Convergence 2021, treatment persistence with JAK inhibitors is comparable to TNF inhibitors and other bDMARDs among patients with rheumatoid arthritis.

Subcategories:AnalgesicsBiologics/DMARDs

According to a German study presented during ACR Convergence 2021, treatment persistence with JAK inhibitors is comparable to TNF inhibitors and other bDMARDs among patients with rheumatoid arthritis.

Enabling rheumatology practices to use complex administration codes for biologic drugs is critical for maintaining patient access to essential therapies.

A larger proportion of patients with gout had a therapeutic response at six months when treated with methotrexate and pegloticase than with pegloticase alone, according to results from the multi-center, open-label MIRROR (methotrexate to increase response rates in patients with uncontrolled gout receiving KRYSTEXXA) study, recently published in the Journal of Rheumatology.1 The MIRROR study…

In a study, patients with new-onset polymyalgia rheumatica (PMR) who were treated with subcutaneous tocilizumab were more likely to achieve sustained, glucocorticoid-free remission than patients who received placebo.
U.S. Food & Drug Administration |
On Dec. 8, the U.S. Food & Drug Administration (FDA) issued an emergency use authorization (EUA) for AstraZeneca’s Evusheld (tixagevimab co-packaged with cilgavimab and administered together) for the pre-exposure prophylaxis (prevention) of COVID-19 in certain adults and pediatric individuals (12 years of age and older weighing at least 40 kilograms [about 88 pounds]). The product…

ACR CONVERGENCE 2021—Rheumatology patients who test positive for COVID-19 would benefit from early use of monoclonal antibodies, said Luis Ostrosky-Zeichner, MD, chief of the Division of Infectious Diseases, McGovern Medical School, University of Texas Health Science Center (UTHealth), Houston, in a session about effective treatment options for COVID-19. Acknowledging that the SARS-CoV-2 virus has already…

Researcher identified multiple factors for flare, including non-use of methotrexate, in patients with rheumatoid arthritis who had switched from intravenous (IV) tocilizumab to subcutaneous tocilizumab.
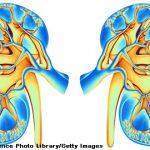
Patients who have undergone a kidney transplant and have high levels of serum uric acid symptomatic gout may benefit from treatment with pegloticase, according to a recent study.

ACR Convergence 2021—The Great Debate at the meeting sparked a thoughtful discussion on the future of lupus nephritis treatment strategies, with experts saying clinicians should be open to new ways of approaching patient care. In the past year, approvals of the monoclonal antibody belimumab and the calcineurin inhibitor voclosporin for use in lupus nephritis (when…

ACR Convergence 2021—The recent Boxed Warning requirement applied to three Janus kinase (JAK) inhibitors, cautioning doctors and patients about several major risks in patients with rheumatoid arthritis, came only after rigorous data collection and careful consideration of the risks and the benefits, U.S. Food & Drug Administration (FDA) officials said in November at ACR Convergence…