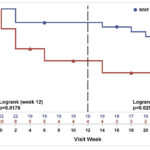
Recent breakthroughs in stem cell-based treatments for arthritis may help delay joint replacement for some patients. Farshid Guilak, PhD, described the methods for creating bioartificial cartilage, its implications for inflammation, disease flare and more.