(Reuters)—Regeneron Pharmaceuticals Inc. said on Tuesday it had identified hundreds of antibodies that could treat or prevent the coronavirus (COVID-19) and was preparing to begin clinical trials by early summer, sending the drugmaker’s shares up 12%. The company will select the top two antibodies to develop a “cocktail” treatment and scale up its manufacturing to…
Sanofi, Regeneron Begin Testing Arthritis Drug as Coronavirus Treatment
(Reuters)—Sanofi SA and partner Regeneron Pharmaceuticals Inc. have started a clinical trial of their rheumatoid arthritis drug Kevzara (sarilumab) as a treatment for the coronavirus, the companies said on Monday. Enrolments for the mid-to-late stage trial will begin immediately, and the companies anticipate the trial will test up to 400 patients. Sarilumab is an infection-fighting…
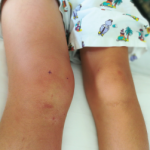
Pediatric Cases Require Special Considerations & Aggressive Treatment Plans
ATLANTA—Managing pediatric patients with rheumatic disease involves special considerations, such as developmental concerns and physiological traits that may affect dosing of medications, according to two experts. During a session at the 2019 ACR/ARP Annual Meeting, Courtney Kremer, ARNP, a pediatric nurse practitioner at the University of Iowa Stead Family Children’s Hospital, Iowa City, and Jessica…
Clinicians Discuss Current & Future Rheumatoid Arthritis Approaches
ATLANTA—When it comes to treating rheumatoid arthritis (RA) patients, most clinicians agree: One size does not fit all. Many treatment options exist, and seldom is there 100% consensus on what the first course of action or general approach should be. In the face of such variability, four clinicians took the stage at the 2019 ACR/ARP…
A Public Health Approach to Arthritis
ATLANTA—Rheumatic diseases have been the subject of a range of public health campaigns and reports over the past decade, but improving their visibility remains a work in progress, a Centers for Disease Control and Prevention (CDC) expert said at the 2019 ACR/ARP Annual Meeting. A growing attention to pain and the opioid crisis may help…

FDA Update: New Drug Approvals, New & Expanded Indications, & More
ATLANTA—New drug approvals, new and expanded drug indications, and important safety and other updates relevant for rheumatologists were presented by three physicians from the U.S. Food & Drug Administration (FDA) on Nov. 11 at the 2019 ACR/ARP Annual Meeting. New JAK Inhibitor Approved for RA On Aug. 16, 2019, the FDA approved upadacitinib (Rinvoq), an…

Risankizumab & Apremilast Come to Market in Canada
In Canada, five provinces will now reimburse patients with plaque psoriasis who use risankizumab. Also, Canada Health has approved apremilast for treating adults with plaque psoriasis and psoriatic arthritis…
Sports Doctors May Accidentally Prescribe Banned Steroids
(Reuters Health)—Sports physicians routinely prescribe corticosteroids to athletes for conditions, such as inflammation, asthma and allergies, but not all of them know which forms of these drugs are banned under anti-doping rules, a study suggests. The survey of 603 physicians from 30 countries found four in five prescribe oral corticosteroids to athletes, one of the…
China Approves Use of Tocilizumab for Coronavirus Patients
BEIJING (Reuters)—China will use a Roche Holding AG arthritis drug to treat some coronavirus patients in severe conditions, health authorities said on Wednesday, as the country seeks to build up treatment regimens to help the infected recover. Tocilizumab, sold by the Swiss pharma giant under the trade name Actemra, can be prescribed to coronavirus patients…

Phase 3 Results for Olokizumab in RA Patients
In a recent study, olokizumab proved safe and effective for treating the signs and symptoms of RA and improving patients’ physical function…
- « Previous Page
- 1
- …
- 29
- 30
- 31
- 32
- 33
- …
- 122
- Next Page »