According to new data, BMS-986165, an oral, selective tyrosine kinase 2 inhibitor, may be safe and effective for treating plaque psoriasis…

Subcategories:AnalgesicsBiologics/DMARDs

According to new data, BMS-986165, an oral, selective tyrosine kinase 2 inhibitor, may be safe and effective for treating plaque psoriasis…
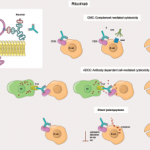
Severe infections occurred in 25% of patients with anti-neutrophil cytoplasm antibody (ANCA) associated vasculitis (AAV) who were treated with rituximab, according to results of an observational study conducted in the United Kingdom (U.K.) and Austria. Trimethoprim-sulfamethoxazole (TMP/SMX) prophylaxis, however, reduced the risk of severe infections, the investigators reported in Annals of the Rheumatic Diseases.1 Andreas…

A patient with rheumatoid arthritis (RA) comes to your office and needs a medication. You prescribe it, and the patient’s insurance plan covers it. The patient begins the medication and slowly but surely feels better. Prescribing drugs for a patient should be this simple but rarely is, thanks to the high cost of drugs and…

A recent study suggests Zilretta, an intra-articular injection for OA knee pain, may lower blood glucose levels in OA patients…

After meeting with the FDA, the makers of intravenous meloxicam plan to resubmit the treatment’s new drug application…
Megan Brooks |
NEW YORK (Reuters Health)—A large epidemiological study provides more evidence of a link between tumor necrosis factor inhibitors (TNFi) and peripheral neuropathy (PN). “These events are rare and the benefit of these drugs still outweigh this risk so this should not change practice,” cautions first author Dr. Mahyar Etminan from the University of British Columbia…
Linda Carroll |
(Reuters Health)—Vitamin D supplementation may not improve bone density or prevent fractures and falls in adults, a large new analysis suggests. After combining data from 81 randomized controlled trials, researchers found no bone benefits from supplementing the vitamin, according to the report in The Lancet Diabetes & Endocrinology, online October 4. “Our results show that…
Will Boggs MD |
NEW YORK (Reuters Health)—Low-dose amitriptyline does not have clear benefits for patients with chronic low-back pain that has no specific cause, according to results from a randomized clinical trial. Despite the lack of evidence that antidepressants are more effective than placebo for low-back pain, seven of 14 national and international guidelines recommend their use in…
Daniel Dickson and Ben Hirschler |
STOCKHOLM/LONDON (Reuters)—Two Americans and a Briton won the 2018 Nobel Prize for Chemistry on Wednesday for harnessing the power of evolution to generate novel proteins used in everything from environmentally friendly detergents and biofuels to cancer drugs. The fruits of this work include the world’s top-selling prescription medicine – the antibody injection Humira sold by…

The FDA has expanded the new Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS) to include immediate release opioids. The program, which also includes extended release and long acting opioids, will provide education to prescribers and healthcare professionals…