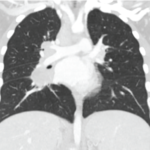
Your patient is deciding whether to enroll in a clinical trial at your institution and wants your advice about whether to participate.
The ACR/CHEST ILD Guidelines in Practice, a video
In collaboration with the American College of Chest Physicians, the ACR released two new comprehensive guidelines aimed at improving the screening, monitoring, and treatment of patients with interstitial lung disease (ILD) secondary to systemic autoimmune rheumatic diseases (SARDs). Recently, Sindhu R. Johnson, MD, PhD, professor of medicine at the University of Toronto, Canada, director of the Toronto Scleroderma Program and principal investigator for the guideline, and Elana J. Bernstein, MD, MSc, Florence Irving associate professor of medicine in the Division of Rheumatology at Columbia University, New York City, and co-first author, presented a webinar to talk about how the guidelines were developed and present some of the recommendations and their rationale: Watch the recording now!
Ethics Forum: Letters from Our Readers—Accepting Gifts
We received a few letters in response to the December 2011 Ethics Forum, which asked the question: What is your personal policy about accepting gifts?
Terminate Staff with Caution
Key principles that will help facilitate terminations that bring cost-effective finality to the employment relationship.
Letter: Another Thinking Discipline
I read today the article “How a Rheumatologist Thinks” and I want to say that is one of the most interesting articles I’ve read about clinical medicine.
CMS Delays ICD-10 Compliance Date
Despite months of assurances to the contrary, the Center for Medicare and Medicaid Services announced in mid-February they were indefinitely postponing implementation of International Classification of Diseases, Tenth Revision, Clinical Modification. The changeover was originally scheduled to take effect October 1, 2013.
First ARHP ‘Best of the Meeting’ Highlights Sleep Research, Osteoporosis Screening, More
“The line between ACR and ARHP sessions has totally blurred,” said Donah Zack Crawford, MA, during the presentation, “Highlights from the 2011 ARHP Sessions,” here at the 2011 ACR/ARHP Annual Scientific Meeting held in Chicago in November 2011.
Biosimilar Drugs Face Challenges to Reach the U.S. Market
To date, the FDA has not approved a biosimilar product. In its own discussion of the merits and obstacles to biosimilar drugs, the ACR sponsored a panel session titled, “Biosimilar Products in the U.S. Market: Fact or Fiction?” at the 2011 ACR/ARHP Annual Scientific Meeting here in November.
A Journey Begins with the First Step
The ACR Research and Education Foundation (REF) has launched the Action Alliance network, a program that calls on rheumatologists, investigators, and health professionals to join the REF in asking patients and families to be a part of the conversation. The Action Alliance consists of two programs working together: From the Field Speakers Bureau and Patients and Families for Progress.
Magnify Rheumatology’s Legislative Impact this Month
Members of the ACR’s Executive, Government Affairs, and RheumPAC Committees, along with the Affiliate Society Council, attended 75 meetings with legislators and their staff. These meetings are critical to advancing awareness of rheumatology and the issues affecting your profession and patients.
ACR’s Advocacy Toolkit
Ways to contact your member of Congress; tips for communicating with your member of Congress; ways to get patients involved; and donloadable briefs, posters, and brochures.
- « Previous Page
- 1
- …
- 258
- 259
- 260
- 261
- 262
- …
- 307
- Next Page »