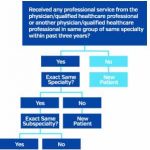
When coding evaluation and management (E/M) services provided to a patient, one of the most persistent concerns is whether a patient is new or established to the practice. Although this may seem like a simple coding answer, the distinction is an important one, because it enables providers to appropriately bill and receive reimbursement correctly. E/M…