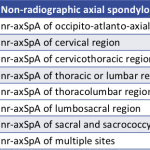
The new diagnostic code should streamline billing for treatment of nr-axSpA, better define the spectrum of spondyloarthritic diseases and enable new research strategies into these conditions.

Larry Beresford |
The new diagnostic code should streamline billing for treatment of nr-axSpA, better define the spectrum of spondyloarthritic diseases and enable new research strategies into these conditions.

In patients with active ankylosing spondylitis, IL-17 inhibitors have produced favorable responses, according to recent research…

ACR BEYOND LIVE—Much, if not all, of rheumatology relies on clinical interpretation of historical, laboratory and imaging information to formulate a coherent diagnosis and treatment plan—even when such information is incomplete or has multiple possible interpretations. One of the best examples of this situation pertains to nonradiographic axial spondyloarthritis (nr-axSpA), a condition that is just…
Reuters Staff |
NEW YORK (Reuters Health)—The ongoing novel coronavirus pandemic has raised concerns about whether biologic therapy could make psoriasis patients more susceptible to SARS-CoV-2, the virus behind COVID-19. Mark Lebwohl, MD, from Icahn School of Medicine at Mount Sinai Hospital in New York and colleagues address this issue in a letter in the Journal of the…

ATLANTA—New drug approvals, new and expanded drug indications, and important safety and other updates relevant for rheumatologists were presented by three physicians from the U.S. Food & Drug Administration (FDA) on Nov. 11 at the 2019 ACR/ARP Annual Meeting. New JAK Inhibitor Approved for RA On Aug. 16, 2019, the FDA approved upadacitinib (Rinvoq), an…

Last year, the FDA was busy with new biologic and other drug approvals, new and expanded drug indications, and important safety updates relevant to rheumatology…

A 2019 update of the ACR’s previous clinical practice guideline on axial spondyloarthritis is now available online. Lead investigator Michael Ward, MD, shares advice for implementing the guideline updates, including those related to sequencing and tapering biologics, and knowing when to obtain images.
If approved by the U.S. Food and Drug Administration (FDA), difficult-to-treat patients with axial spondyloarthritis who fail or are intolerant to standard treatment with tumor necrosis factor inhibitors (TNFi) may have a new treatment option. That new option is a high-affinity monoclonal antibody, called ixekizumab, which selectively targets an area linked to the immunopathology of…

Recent research indicates that low-density neutrophils, such as low-density granulocytes, exert proinflammatory effects on the T cells of SLE patients. In the study, researchers confirmed SLE patients had a higher prevalence of low-density granulocytes than healthy controls and that these cells appeared to promote a Th1 response…
Allison Martell & Allison Lampert |
TORONTO/MONTREAL (Reuters)—The Canadian province of British Columbia said on May 28 that its public drug plan will switch as many as 20,400 patients from three branded biologic drugs to cheap near-copies called biosimilars, saving an estimated C$96.6 million ($71.9 million) over three years. The new policy from the province’s PharmaCare program targets Johnson & Johnson’s…