Spurred by a recent Cigna policy, the ACR will lead a resolution to oppose insurance companies providing financial incentives for patients to switch treatments. Several specialties are cosponsoring the resolution for a special meeting of the AMA House of Delegates in June.
Search results for: Certolizumab pegol

Cigna Offers Patients Financial Incentive to Switch Treatments
The ACR has sent a letter to Cigna expressing opposition to the initiative, which jeopardizes patients’ health, interferes with medical decision making, undermines the doctor-patient relationship and may disproportionately affect patients of lower socioeconomic status.
Cytokine Targets & Treatment Developments for Psoriatic Arthritis & Spondyloarthritis
ACR CONVERGENCE 2020—In recent years, a pathophysiological role for the interleukin (IL) 17/IL-23 axis in the development of psoriasis, enthesitis and inflammatory arthritis has been investigated in both rodent and human models. Clinical trials have demonstrated differential benefits for skin disease and joint disease in patients with psoriatic arthritis, axial spondyloarthritis (axSpA) and ankylosing spondylitis…

A Year to Remember: Dr. Jinoos Yazdany Presents the 2020 Clinical Research Review
During ACR Convergence 2020, Jinoos Yazdany, MD, MPH, discussed innovative research into a potential treatment for lupus, medication tapering & more. These findings may influence the treatment of rheumatic disease in the future.
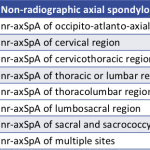
Non-Radiographic Axial Spondyloarthritis Recognized with ICD-10 Code
The new diagnostic code should streamline billing for treatment of nr-axSpA, better define the spectrum of spondyloarthritic diseases and enable new research strategies into these conditions.

FDA Update: New Drug Approvals, New & Expanded Indications, & More
ATLANTA—New drug approvals, new and expanded drug indications, and important safety and other updates relevant for rheumatologists were presented by three physicians from the U.S. Food & Drug Administration (FDA) on Nov. 11 at the 2019 ACR/ARP Annual Meeting. New JAK Inhibitor Approved for RA On Aug. 16, 2019, the FDA approved upadacitinib (Rinvoq), an…

FDA Rheumatology Update: New Drug Approvals, Plus Expanded Drug Indications & Safety Concerns
Last year, the FDA was busy with new biologic and other drug approvals, new and expanded drug indications, and important safety updates relevant to rheumatology…

RA Patients May Be Less Likely to Discontinue Etanercept Than Other TNF Inhibitors
In a systematic literature review, researchers found that rheumatoid arthritis patients taking etanercept were less likely to discontinue their treatment than patients using any of five other tumor necrosis factor inhibitors…

Rheumatology Drugs at a Glance, Part 3: Rheumatoid Arthritis
Over the past few years, biosimilars and other new drugs have been introduced to treat rheumatic illnesses. Some of the conditions we treat have numerous drug options, others have few or only off-label options. This series, “Rheumatology Drugs at a Glance,” provides streamlined information on the administration of biologic, biosimilar and small molecule inhibitor drugs…

Biological DMARDs in Elderly RA Patients: Use, Maintenance & Discontinuation
A study comparing seven biologic DMARDs in RA patients aged 65 years and older found abatacept had the highest retention rate and the lowest discontinuation rate…
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- 6
- …
- 8
- Next Page »