NEW YORK (Reuters Health)—Patients with some inflammatory rheumatic conditions are at higher risk for hospital-diagnosed COVID-19 infection compared with the general population, but it depends on the condition and therapy used to treat it, according to a study from Spain. It’s now clear that older patients and those with some common diseases are at increased…
Search results for: hospital

COVID-19: Most Individuals with Rheumatic Disease Recover
An analysis of data from the COVID-19 Global Rheumatology Alliance registry shows that use of disease-modifying antirheumatic drugs or non-steroidal anti-inflammatory drugs did not increase the risk of hospitalization for COVID-19 patients with rheumatic disease, but steroid use did.

Tomisaku Kawasaki, Pediatrician Who Discovered Disease That Bears His Name, Dies at 95
Japanese pediatrician Tomisaku Kawasaki, MD, who identified an inflammatory syndrome that affects children, died on June 5 in Tokyo. He was 95. Tenacity & Attention to Detail Born Feb. 7, 1925, in Tokyo, Dr. Kawasaki graduated from medical school at what is now Chiba University in Chiba, Japan, in 1948 and worked as staff pediatrician…
More Evidence Links Pediatric Inflammatory Multisystem Syndrome to SARS-CoV-2
NEW YORK (Reuters Health)—Two new reports in JAMA strengthen the link between SARS-CoV-2 infection and pediatric inflammatory multisystem syndrome (PIMS). Pediatricians from several communities have reported children who developed fever and multisystem inflammation during the COVID-19 pandemic. Some children were critically ill and some had characteristics similar to Kawasaki disease or Kawasaki disease shock syndrome….
After Hip Fracture, Earlier Osteoporosis Drug Initiation Tied to Lower Subsequent Fracture Risk
(Reuters Health)—Patients hospitalized for a hip fracture are less likely to experience a subsequent fracture-related hospitalization if they start anti-osteoporosis medication sooner, a Taiwanese study suggests.1 Researchers examined data on 77,930 patients aged 50 years and older hospitalized for hip fractures, including 9,986 people prescribed anti-osteoporosis medications within one year of the index fracture. Compared…

The ACR on Air Podcast’s COVID-19 Series
In light of the current COVID-19 pandemic, the ACR has dedicated a few episodes of the ACR on Air podcast to the challenges that have arisen, along with information about the available resources and guidance. The first episode in this special series looks at drug shortages surrounding hydroxychloroquine (HCQ) and its initial uses to treat…

Dr. Bernhard Helps Doctors in Underserved Areas Via the MAVEN Project
In 2018, Gerson Bernhard, MD, FACP, MACR, received a call from a primary care physician at a rural clinic in Florida who was treating patients with varying degrees of arthritis. One patient’s case was more complex than the others. Dr. Bernhard guided the doctor through the patient’s history, reviewed lab results, referred related studies, expanded…
Ustekinumab for Behçet’s Disease? The Study Results Are In
In a multicenter, prospective, open-label study, ustekinumab therapy was effective in treating oral ulcers resistant to colchicine in patients with Behçet’s disease, according to study author David Saadoun, MD, PhD, Department of Internal Medicine and Clinical Immunology, Sorbonne University, Paris, and fellow researchers.1 Researchers focused on the topic because oral ulcers are often disabling, have…
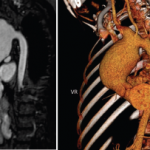
Case Report: Which Vasculitis Is It?
A 13-year-old, adopted girl of unknown ancestry with social anxiety, selective mutism and Takayasu arteritis presented for evaluation of severe, painful, gingival hyperplasia, which limited her oral intake and resulted in weight loss. The young patient was diagnosed with Takayasu arteritis at age 8, when she presented with a persistently elevated erythrocyte sedimentation rate (ESR),…

The History of Treating Lupus with Hydroxychloroquine
Given how unexpectedly front and center hydroxychloroquine has been in discussions about the treatment of COVID-19 this year, it makes sense to look at how it became so central to the treatment of a rheumatologic condition. In 1991, an article appeared in The New England Journal of Medicine that would alter the way rheumatologists approached…
- « Previous Page
- 1
- …
- 102
- 103
- 104
- 105
- 106
- …
- 331
- Next Page »