NEW YORK (Reuters)— Consumer sign-ups for Obamacare individual insurance plans were more than 600,000 during the first week of enrollment for 2018, a U.S. health agency said on Thursday, a positive sign for insurers who take part in the healthcare program that Republicans are trying to undo. The Centers for Medicare & Medicaid Services, a…
Search results for: hospital

Rheumatic Disease Does Not Preclude Pregnancy
Preconception planning is essential to help women with autoimmune disease have optimal pregnancy outcomes. Unplanned pregnancy can also negatively impact disease course in some patients. Yet many rheumatologic patients of childbearing age do not receive adequate contraception or prepregnancy education and counseling. Rheumatologists must work collaboratively with other healthcare providers to make sure rheumatic patients…
Maine Governor Will Not Expand Medicaid, Ignoring Voters
(Reuters)—Maine Republican Governor Paul LePage said on Wednesday he will not expand the state’s Medicaid program under Obamacare, ignoring a ballot initiative widely backed by voters, calling it “ruinous” for the state’s budget. Maine looked set to become the first state in the nation to expand Medicaid by popular vote. About 60% of voters in…
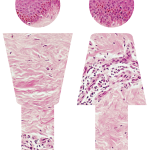
Systemic Sclerosis Mortality Rate May Be Underestimated
Systemic sclerosis (SSc) is a disease characterized by immunologic abnormalities, microvascular involvement and tissue fibrosis. In previous studies, 10-year survival rates ranged from 50–84%. However, there are concerns that these studies, using prevalent cohorts, are underestimating mortality. “While the prognosis of many rheumatic diseases has improved with the availability of more effective and targeted therapies,…

Unwise Choices: EHRs, PBMs, Drug Costs Are Leading to Physician Burnout
My dear electronic health records How do I dislike thee? Let me count the ways Adaptation of Sonnet 43 By Elizabeth Barrett Browning, 1806–1861 As my tenure as physician editor winds down, it’s worth reviewing some of the more nettlesome issues confronting clinicians that have been previously discussed in these pages and gauge their current…

Ustekinumab Approved for Use in Adolescents with Plaque Psoriasis
In October, the FDA approved ustekinumab to treat patients 12 years or older who have moderate to severe plaque psoriasis…
Puerto Rico Seeks Help as Medicaid Crisis Deepens after Maria
WASHINGTON/SAN FRANCISCO (Reuters)—Puerto Rico, still reeling from Hurricane Maria, is asking the Trump administration and U.S. lawmakers for help in staving off a Medicaid crisis that has put a quarter of the island’s residents at risk of losing medical care. The territory, which has grappled for years with shortfalls in funding of its Medicaid healthcare…

One Member’s Personal Story Illustrates How RheumPAC Can Help Underserved Children
Almost one year into my term on the ACR’s Political Action Committee, RheumPAC, I’m looking back and reflecting on what led me here. My early career was focused on learning the basics of clinical practice and building relationships with healthcare providers. Shortly after I completed my fellowship, I established goals to improve access to care…
Lupus Survival Is Improving Slowly
NEW YORK (Reuters Health)—Systemic lupus erythematosus (SLE) mortality has declined during the past 46 years in the U.S.—but more slowly than mortality in the general population, according to a nationwide study. “Based on our experience in the clinic and according to previous reports showing improvement in the short-term (five- to 10-year) survival in lupus, I…

FDA Sets Stricter Requirements for Immediate-Release Opioids to Prevent Misuse & Abuse
The FDA is expanding its Risk Evaluation and Mitigation Strategy (REMS) to include manufacturers of immediate-release opioids. The makers of these drugs will soon be required to provide training and education to healthcare professionals on the proper prescription and use of the drugs for pain management…
- « Previous Page
- 1
- …
- 165
- 166
- 167
- 168
- 169
- …
- 320
- Next Page »