NEW YORK (Reuters Health)—Racial and ethnic disparities in systemic lupus erythematosus (SLE) incidence and prevalence are considerable, according to two new studies of data from California and Manhattan. “The most important finding of the population-based California and New York registries is the confirmation of the racial and ethnic disparities of SLE, with the highest incidence…
Search results for: hospital
State Funding Changes in Spotlight in U.S. Republican Healthcare Bill
WASHINGTON (Reuters)—Republican leaders sought to nail down the final votes needed to pass what U.S. Vice President Mike Pence on Thursday called their “last best chance” to repeal Obamacare while a new analysis underscored how Democratic-leaning states stand to lose large amounts of federal funding under the legislation. Senate Majority Leader Mitch McConnell (R-Ky.) plans…
Inflammatory Spinal Disorders Common in IBD Patients
NEW YORK (Reuters Health)—Ankylosing spondylitis (AS), axial spondyloarthritis (SpA) and inflammatory back pain are common in inflammatory bowel disease (IBD) patients two decades after IBD diagnosis, according to findings from the IBSEN study1. Doctors should know IBD patients are at risk of inflammatory back problems, and refer them to a rheumatologist when appropriate, Dr. Alvilde Ossum…
Pfizer Files Suit Against J and J over Remicade Contracts
(Reuters)—Drugmaker Pfizer Inc on Wednesday filed a lawsuit against Johnson and Johnson, saying its rival’s contracts with health insurers for blockbuster rheumatoid arthritis drug, Remicade, were anticompetitive and blocked sales of Pfizer’s new biosimilar. Pfizer said in the suit that Johnson and Johnson is offering discounts on its Remicade treatment in exchange for essentially excluding…
Unbudgeted: How the Opioid Crisis Is Blowing a Hole in Small-Town America’s Finances
INDIANA, Pa./CHILLICOTHE, Ohio (Reuters)—As deaths mount in America’s opioid crisis, communities on the front lines face a hidden toll: the financial cost. Ross County, a largely rural region of 77,000 people an hour south of Columbus, Ohio, is wrestling with an explosion in opioid-related deaths—44 last year compared with 19 in 2009. The drug addiction…
U.S. Democrats Urge Full Review Before Senate Vote on Latest Obamacare Attack
WASHINGTON (Reuters)—Democratic leaders in the U.S. Congress on Monday demanded that lawmakers wait to find out the budgetary and healthcare impacts of a new, last-ditch legislative effort by Republicans to repeal Obamacare before voting on it. In their long-running war on former President Barack Obama’s signature healthcare law, Senate Republicans are now proposing to replace…
TNF Inhibitor Drug Tapering Successful in Some Patients with RA
MADRID—Scores on the Health Assessment Questionnaire for Rheumatoid Arthritis (HAQ) and C-reactive protein (CRP) levels were independent predictors of whether patients could be tapered successfully from a TNF inhibitor after having reached remission of their RA, according to findings presented in a session at the Annual European Congress of Rheumatology. Researchers also developed a composite…
Rheumatoid Arthritis Treatments Show Mixed Results
MADRID—The anti-IL6 “nanobody,” vobarilizumab, produced mixed results when used with methotrexate (MTX) and compared with MTX and placebo, according to results of a 24-week, double-blind Phase 2b study of patients with rheumatoid arthritis (RA), which were presented in an abstract session at the Annual European Congress of Rheumatology (EULAR). The drug missed its primary endpoint…

Skype-Based Biopsychosocial Treatments Help Save Physical Therapy Patients Time, Trouble
It’s a bit ironic that when injured people are in pain—and their mobility is reduced—they are often expected to travel to a physical therapy clinic. For millions of people, such trips are a burden. In Australia, however, some patients are “letting movement come to them.” Novel research from The University of Melbourne shows that taking…
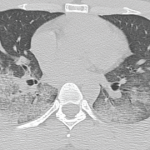
Hemoptysis in a Young Indian Male
A 22-year-old Indian male presented to the emergency department with hemoptysis. A month prior, he had presented to an urgent care center complaining of cough with occasional episodes of blood-tinged sputum in the morning. He was diagnosed with community-acquired pneumonia based on a chest X-ray without laboratory testing and was prescribed levofloxacin. A few days…
- « Previous Page
- 1
- …
- 181
- 182
- 183
- 184
- 185
- …
- 331
- Next Page »