The FDA is investigating the safety of MRIs using gadolinium-based contrast agents, which recent studies have shown may leave deposits of those chemicals in patients’ brain tissue after multiple scans…
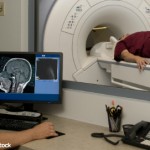

The FDA is investigating the safety of MRIs using gadolinium-based contrast agents, which recent studies have shown may leave deposits of those chemicals in patients’ brain tissue after multiple scans…
Ben Hirschler |
LONDON (Reuters)—The Chinese market is getting tougher for Western pharmaceutical companies as Beijing bears down on a rising healthcare bill and prices come under pressure. The country, which has overtaken Japan as the world’s second largest market for prescription medicines after the U.S., has drawn major investment from global drugmakers in recent years — but…
Rob Goodier |
NEW YORK (Reuters Health)—Two classes of inflammatory diseases, uveitis and psoriatic disease, appear linked, as a diagnosis of one increases the risk of developing the other, new research has found. A study of Danish patient registries found nearly triple the rate of uveitis among patients with psoriatic arthritis compared to the general population, and double…
Ransdell Pierson |
(Reuters)—Closer coordination between healthcare facilities and public health departments could save 37,000 U.S. lives over five years by preventing infections from antibiotic-resistant germs and from Clostridium difficile, according to a government report released on Tuesday. Germs that no longer respond to antibiotics cause more than 2 million illnesses and 23,000 deaths each year in the…

Phase 2 clinical trials have begun to assess the safety of brentuximab vedotin for the treatment of SLE. Also, the FDA is reviewing an application for a once-daily tofacitinib citrate tablet to treat RA…
Reuters Staff |
BEIJING (Reuters)—China will expand medical insurance to cover all critical illnesses for all urban and rural residents by the end of the year, the cabinet said on Sunday, the latest step in a plan to fix a healthcare system that has sparked public discontent. The State Council said 50% of the medical costs will be…
Susan Kelly |
(Reuters)—Insurer CareFirst BlueCross BlueShield said on Thursday its cost savings on providing healthcare rose sharply last year in a program that rewards doctors for keeping patients out of the hospital. The non-profit health insurer operates an approach to delivering care that emphasizes coordination among providers, led by a patient’s primary care physician. The model is…
Reuters Staff |
NEW YORK (Reuters Health)—Dermatologic complications hit about one in five patients with inflammatory bowel disease (IBD) on anti-tumor necrosis factor (anti-TNF) therapy, leading to discontinuation of treatment, a French study finds. Dr. Laurent Peyrin-Biroulet, from University Hospital of Nancy, and colleagues note that dermatological complications of anti-TNF therapy are known to occur frequently in IBD…
Will Boggs, MD |
NEW YORK (Reuters Health)—The American Board of Internal Medicine (ABIM) maintenance-of-certification (MOC) program could cost $5.7 billion in physicians’ time and fees over the next decade, according to a new model study. “We estimate that physicians will spend 33 million hours over 10 years to fulfill MOC requirements,” Dr. Dhruv S. Kazi from the University…
Caroline Humer |
NEW YORK (Reuters)—The U.S. government expects healthcare spending to increase by 5.8% annually on average from 2014 through 2024 as more Americans gain insurance coverage and the improved economy drives patients to visit doctors and hospitals. The aging population’s higher healthcare costs will also push health spending higher starting in 2019, according to a study…