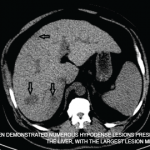
Case Presentation A 45-year-old man with crystal-proven gout, poorly controlled diabetes and chronic kidney disease was lost to follow-up for six years and presented back to the VA clinic in the midst of a gout flare. He stated he had continued taking 100 mg of allopurinol daily, but his serum urate level was 13.8 mg/dL….