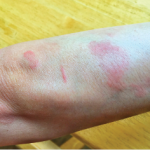
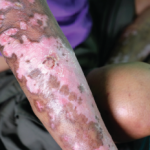
CHICAGO—When Richard Furie, MD, was first asked to speak about lupus at the 2019 ACR State-of-the-Art Clinical Symposium, held April 5–7, organizers suggested he discuss low disease activity and classification criteria. But Dr. Furie, a professor of medicine at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, N.Y., and a veteran investigator…