The ACR is compiling a list of healthcare policy changes relevant to rheumatology providers and patients that will take effect when the public health emergency expires on May 11.
COVID-19 EUA Testing Requirement Change
On Feb. 3, the U.S. Food & Drug Administration (FDA) revised its Letters of Authorization for two emergency use authorizations (EUAs), Paxlovid and Lagevrio, to remove the requirement for positive test results to prescribe these drugs. The agency continues to recommend that providers use direct SARS-CoV-2 viral testing to help diagnose COVID-19. The FDA recognizes…

What We Know about COVID-19 in 2023: Variants, Vaccines, New Therapies & More
Although a less central focus than it was three years ago, rheumatologists must still consider the prevention and management of SARS-CoV-2 in their patients. The following update shares ongoing considerations related to the COVID-19 pandemic. Outcomes At the beginning of the pandemic, it was unclear whether patients with rheumatic disease would be at higher risk…

Long COVID: Experts Weigh in on Increasingly Common Syndrome
A minority of patients experience lingering symptoms after infection with SARS-CoV-2, similar to some other previously known post-infection syndromes. Although we are just beginning to understand the different presentations, pathophysiology, risk factors, prognosis and treatment of long COVID, rheumatologists can play a leadership role in managing patients with the illness and contributing to this important research…
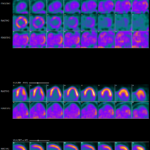
Case Report: Is It Cardiac Sarcoidosis or COVID-19 Myocarditis?
COVID-19 causes myriad cardiac dysfunctions, ranging from mild to fulminant disease, including myocarditis, acute congestive heart failure, cardiogenic shock and sudden cardiac death.1,2 COVID-19 myocarditis can mimic cardiac sarcoidosis clinically and on cardiac imaging, which can lead to diagnostic challenges and treatment delays. We present a case of cardiac sarcoidosis with interval development of metabolic…

ACR Convergence 2022 Closing Session Discusses Research Highlights
PHILADELPHIA—Expert panelists gathered in the closing session at ACR Convergence 2022 to give their take on what they saw as some of the most notable research findings and other insights to come out of the meeting, touching on a number of topics on the leading edge of the field. COVID-19 Prophylaxis & Vaccinations Alfred Kim,…
FDA Revokes Emergency Use Authorization for Evusheld
The Food & Drug Administration announced Jan. 26 that tixagevimab/cilgavimab (Evusheld) is no longer authorized for use in the U.S. The decision was based on new data suggesting that the treatment is unlikely to be active against the most common current SARS-CoV-2 variants.

In Memoriam: A Tribute to Dr. Philip Robinson
We write to celebrate the life of Philip C. Robinson, MB ChB, PhD, FRACP, a beloved colleague and leader in rheumatology. Phil died in early January after an unexpected and short illness. He is survived by his wife, Helen, and his two young sons. We have witnessed an incredible outpouring of respect and affection for…
Gout & Excess Risk of Severe SARS-CoV-2 Infection
In this large, population-based study, Xie et al. found that the risks of SARS-CoV-2 infection, 30-day hospitalization and 30-day death were higher among individuals with gout than individuals without gout in the general population, irrespective of COVID-19 vaccination status.
COVID-19 Vaccine Responses Among the Immunosuppressed
PHILADELPHIA—Patients with rheumatic diseases often mount an adequate immune response after receiving COVID-19 vaccinations, but that is not always the case, and certain medications make patients more prone to having an insufficient response, said Judith James, MD, PhD, chair, Arthritis and Clinical Immunology Research Program, Oklahoma Medical Research Foundation, Oklahoma City, at an ACR Convergence…
- « Previous Page
- 1
- 2
- 3
- 4
- …
- 27
- Next Page »