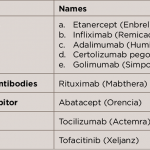
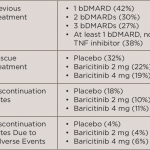
WASHINGTON, D.C.—Clinical aspects of managing patients with rheumatoid arthritis (RA) in remission were discussed by a panel of experts at the 2016 ACR/ARHP Annual Meeting during the session titled Rheumatoid Arthritis—Clinical Aspects IV: Managing Patients in Remission. Among the issues raised were strategies to taper or discontinue biologic therapies, as well as clinical predictors of…