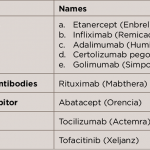
A systematic review has found that anti-tumor necrosis factor alpha treatment may improve endothelial function in RA patients. Despite the heterogeneity of the included studies, a random-effects model showed a significant improvement in endothelial function in this patient population after receiving infliximab, adalimumab or etanercept…