NEW YORK (Reuters)—Pacira Pharmaceuticals Inc. on Tuesday filed a lawsuit seeking a court order allowing it to promote its post-surgery pain drug, Exparel (bupivacaine liposome injectable suspension), for a wide range of surgeries, which the U.S. Food and Drug Administration opposes. The lawsuit, filed in federal court in Manhattan, cites another New York judge’s recent…

Lupus Nephritis Therapies Compared, Plus Naming Guidance for Biosimilars
Comparing tacrolimus, mycophenolate mofetil and cyclophosphamide, tacrolimus was the most efficacious. Also, biosimilars may soon be easier to differentiate…
FDA Warns of Severe Joint Pain Risk with DPP-4 Diabetes Drugs
(Reuters)—A class of diabetes drugs that include Merck & Co Inc.’s Januvia has been linked with severe joint pain, the U.S. Food and Drug Administration said on Friday. The FDA said it had identified 33 cases of severe joint pain in patients taking a class of drugs known as DPP-4 inhibitors between Oct. 16, 2006,…
FDA Proposes Adding Suffixes to Distinguish Biosimilar Drug Names
WASHINGTON (Reuters)—The U.S. Food and Drug Administration proposed on Thursday identifying cheaper versions of biologic drugs with a suffix to distinguish them from their more expensive, branded counterparts. The FDA said its draft guidance is designed to prevent the inadvertent substitution of non-interchangeable products and to make it easier to monitor and track usage once…
FDA Warns Makers of Superbug-Prone Scopes over Testing Violations
(Reuters)—Manufacturers of duodenoscopes linked to recent superbug outbreaks at U.S. hospitals skirted a host of testing, manufacturing and reporting requirements, the U.S. Food and Drug Administration said in warning letters to the companies released on Monday. The letters, sent on Aug. 12, cite Olympus Corp Pentax Medical and Fujifilm Holdings Corp with multiple violations found…
High-Risk Medical Devices Backed by Few Studies
(Reuters Health)—Many high-risk therapeutic devices get U.S. Food and Drug Administration (FDA) approval with only one study proving their safety and efficacy before going to market. Studies of how the devices work once they are on the market are also few and far between, according to a new study that looked at all 28 high-risk…
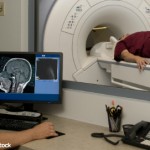
FDA Investigates MRI Safety after Studies Find GBCA Deposits in Brain
The FDA is investigating the safety of MRIs using gadolinium-based contrast agents, which recent studies have shown may leave deposits of those chemicals in patients’ brain tissue after multiple scans…

Brentuximab Vedotin Enters Phase 2 Trials & More
Phase 2 clinical trials have begun to assess the safety of brentuximab vedotin for the treatment of SLE. Also, the FDA is reviewing an application for a once-daily tofacitinib citrate tablet to treat RA…
New Analysis Underscores Improving Pharma R&D Productivity
LONDON (Reuters)—Drug industry productivity is continuing to improve, with a bumper haul of new products being launched and companies proving more successful in the final stages of clinical testing, according to a new analysis. Data from Thomson Reuters published on Tuesday showed the number of innovative medicines, or new molecular entities, launched globally in 2014…

FDA Issues Stronger NSAIDs Warning
The FDA revised its warning and labeling recommendations for antiinflammatory drugs because of a greater understanding of the increased risks they pose for stroke and myocardial infarction…
- « Previous Page
- 1
- …
- 21
- 22
- 23
- 24
- 25
- …
- 29
- Next Page »