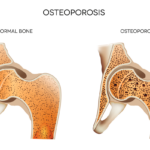
The ACR recently released an update on the prevention and treatment of glucocorticoid-induced osteoporosis.1 The guideline, which includes information on the new therapies abaloparatide and romosozumab, emphasizes the importance of shared decision making by patients and clinicians, and also gives information on the importance of sequential therapy after stopping certain osteoporotic prevention therapies. Fracture Prevention…