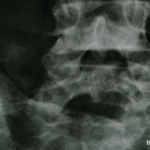
If approved by the U.S. Food and Drug Administration (FDA), difficult-to-treat patients with axial spondyloarthritis who fail or are intolerant to standard treatment with tumor necrosis factor inhibitors (TNFi) may have a new treatment option. That new option is a high-affinity monoclonal antibody, called ixekizumab, which selectively targets an area linked to the immunopathology of…