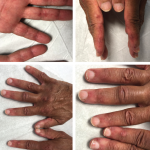
Clinically amyotrophic dermatomyositis (CADM), a subset of dermatomyositis (DM), is a rare autoimmune disease characterized by typical DM cutaneous findings (e.g., heliotrope rash, Gottron papules, Gottron sign) without evidence of myositis.1 The incidence of DM and CADM is approximately 9.63 per 1 million people and 2.08 per 1 million people, respectively.2 The association with development…