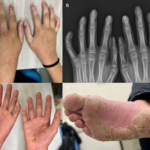
For the 2024 Image Competition, the ACR sought images with educational or remarkable manifestations representing a diverse range of pediatric patients with autoimmune, inflammatory, infectious and malignant drivers of rheumatic disease. Here, we showcase the winning images from Latin America and the Caribbean. Diagnosing Juvenile PsA A 13-year-old boy presented with a seven-year history of…