In a new study, patients taking denosumab had greater treatment adherence over two years than patients on alendronate and other anti-osteoporosis agents…


In a new study, patients taking denosumab had greater treatment adherence over two years than patients on alendronate and other anti-osteoporosis agents…
Will Boggs, MD |
(Reuters Health)—Many drugs granted accelerated approval by the U.S. Food and Drug Administration (FDA) lack clear evidence of safety and effectiveness, and the same is true for most high-risk medical devices, according to two new reports in JAMA, online Aug. 15. The Accelerated Approval pathway makes potentially promising investigational medicines available for use before the…

Opana ER Pulled from U.S. Market Last month, the U.S. Food and Drug Administration (FDA) asked Endo Pharmaceuticals to remove oxymorphone hydrochloride extended release (Opana ER) from the U.S. market due to public health consequences related to abuse. The agency has concerns that the risks presented by the treatment do not outweigh its benefits.1 On…

Romosozumab’s Future Is Uncertain Romosozumab, which has the possible U.S. brand name Evenity, is awaiting approval from the FDA.1 The treatment is an investigational, injectable biologic for treating osteoporosis. It increases bone formation and bone density, reducing a patient’s risk of fractures. The manufacturer no longer expects the FDA to approve the drug this year…

Charles Radis, DO |
First Appearances I watched the old man, his back painfully bent, shuffle toward the scale. A blocky rigidity draped over him. His feet seemed stuck to the floor. His head hung heavily over his chest. Observing him from the end of the hallway, instead of a face, I saw only a mound of shaggy, matted…

While members of Congress debate healthcare legislation, rheumatologists say many of their patients struggle to afford everything from generic drugs to insurance copayments for physical therapy. “It’s a mess. The cost of prescriptions and the rationale for those rising costs in the U.S. right now—it’s just a mess,” says James R. O’Dell, MD, Stokes-Shackleford Professor of…

Bruce N. Cronstein, MD |
Like many people, I am up early and in the gym most days. Although I don’t seem to get anywhere new on the stationary bicycle or the elliptical machine, I do get to keep up with the pundits on the early morning talk shows. In contrast to the television series I binge on later in…

Recent research analyzed factors influencing the selection of the first-line biologic medications and the real-life factors that lead to switching from those medications to other biologics in treating rheumatoid arthritis (RA). The study compared the use of abatacept and tocilizumab with a tumor necrosis factor alpha inhibitor (TNFi).1,2 Participants were enrolled in the Lombardy Rheumatology…
Lina El Kibbi |
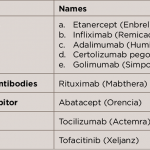
In the past 15 years, rheumatoid arthritis (RA) has posed an economic burden on patients in Saudi Arabia due to the high cost of the medications used to treat the condition. As a rheumatology consultant, I’ve observed the economic impact on patients in one clinic in a private hospital in Riyadh. RA is a chronic,…

Kelly April Tyrrell |
Guselkumab Improves Active Psoriatic Arthritis New research has revealed that patients with active psoriatic arthritis (PsA) and ≥3% body area of plaque psoriasis benefit from treatment with a human monoclonal antibody known as guselkumab (GUS). GUS is specific for the p19 subunit of interleukin 23 (IL-23). Patients in the Phase 2 clinical trial experienced significant…