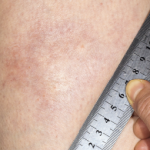
In September 2018, the U.S. Food & Drug Administration (FDA) granted fast-track status to FCX‑013, a gene therapy product developed to treat moderate to severe localized scleroderma (morphea). Previously, the treatment received an orphan drug designation for localized scleroderma, as well as a rare pediatric disease designation. Phase 1 and 2 studies will assess safety…