Take the challenge. CPT: 99214-25, 96413, 96375, 96361-59, J1745 x 4 J1745 JW* x 36, J1200 x1 ICD-10: M45.09, T50.995A, R06.02, E66.3, Z68.2 Rationale Modifier 25 is appropriate to use because it indicates the patient received a significant, separately identifiable E/M service on the same day as the infliximab infusion. This E/M service entailed the…
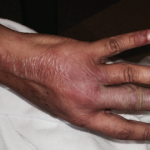
Rheumatology Case Report: Bullous Lesions in Patient with Lupus
Systemic lupus erythematosus (SLE) is a heterogeneous disease associated with multiple acute or chronic cutaneous manifestations, including the relatively rare category of bullous lupus. The development of vesiculo-bullous lesions may be associated with a high morbidity, hence they warrant an urgent investigation, including a skin biopsy to identify the diagnosis and initiate prompt treatment. With…

Biosimilar Drugs Raise Questions around Treatment Efficacy, Quality, Safety
WASHINGTON, D.C.—Challenges abound for the manufacturing of biosimilar drugs—from their sheer size compared with small molecule drugs to the unknowable proprietary aspects of the originator drugs—an expert said at the 2016 ACR/ARHP Annual Meeting in a session titled Immunology Update: Biologic Agents: From Nature to Protein Engineering to Biosimilars. Above all, because biosimilars are copies…

Straightforward Approach Can Help Rheumatology Health Professionals Engage with Fibromyalgia Patients
“I have pain all over my body” is a challenging response after you’ve asked a new patient what brings them in for their visit. You immediately suspect that this patient has fibromyalgia. The prevalence of fibromyalgia in the U.S. is 5 million people, and it is among the most common conditions in many rheumatology practices….

Rheumatologists, Social Workers Collaborate to Help Patients with Lupus
At the Hospital for Special Surgery (HSS), New York, rheumatologists and social workers have found that an interdisciplinary approach to care for systemic lupus erythematosus (SLE) patients improves the overall patient experience. “Our goal is to help patients navigate the complex healthcare system,” says Jillian Rose, LCSW, MPH, assistant director, Community Engagement, Diversity & Research….

Diagnosis of Acute Gouty Arthritis Obscured by Anchoring Bias
A 56-year-old African American man presents to the emergency department with polyarthralgias and a fever of 103ºF. One month prior to admission, he presented with right knee pain and swelling. Blood cultures grew S. epidermidis. He was treated for presumed septic arthritis complicated by MSSE bacteremia. He was treated with meropenem and a prolonged course…

How to Bill Medicare Patients for Non-Covered Services
What do you do when you are presented with a patient who needs treatment but the patient’s insurance company will not pay for the services? Can you provide the services anyway? Who will pay for them? How do you collect payment for such services? If the patient consents to receive the services in spite of…
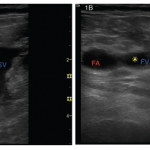
Rheumatology Case Report: Deep Vein Thrombosis Detected by Point-of-Care Ultrasound
Case A 46-year-old Caucasian female presented to the outpatient rheumatology clinic where she had been followed for several years. Her chief complaint was pain in her right knee, posterior right thigh and right hip that had begun gradually over the previous three weeks. Her past medical history was significant for rheumatoid arthritis (RA), obesity and…

Use Time Component When Coding Counseling, Coordination of Care Visits
Although there are seven components for the levels of evaluation and management (E/M) services, most encounter levels are coded on the basis of the history, examination and medical decision making (MDM), which are the key components extracted from documentation in the medical record. However, when counseling and coordination of care for a patient are the…

Participate in Virtual Capitol Hill Meetings with the ACR
On May 11, ACR leadership representatives from the Board of Directors, the Affiliate Societies Council and the Committee on Government Affairs and RheumPAC will take the ACR’s policy messages to Capitol Hill for the Advocacy Leadership Conference. There is power in numbers, so we hope you will participate in our Virtual Hill Day by visiting…
- « Previous Page
- 1
- …
- 19
- 20
- 21
- 22
- 23
- …
- 86
- Next Page »