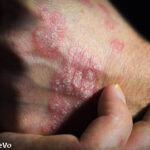
Results from two studies found that deucravacitinib improved the signs and symptoms of patients with psoriatic arthritis who were biologic disease-modifying anti-rheumatic drug naive and those previously treated with a tumor necrosis factor α inhibitor.