Subcutaneous tocilizumab is the first biologic agent approved by the FDA treat patients with systemic sclerosis-associated interstitial lung disease.


Subcutaneous tocilizumab is the first biologic agent approved by the FDA treat patients with systemic sclerosis-associated interstitial lung disease.
Reuters Staff |
NEW YORK (Reuters Health)—Barrett’s esophagus is common in women with systemic sclerosis or scleroderma (SSc) and is often accompanied by dysplasia, according to the largest study on prevalence of Barrett’s esophagus in women with SSc. In Barrett’s esophagus, chronic gastric reflux causes the lining the esophagus to be replaced by metaplastic cells that may lead…
A recent paper in Arthritis & Rheumatology opens up the possibility of a new research avenue to treat systemic sclerosis: dipeptidyl peptidase 4 (DPP4) inhibitors, a previously approved therapy for type 2 diabetes.1 Work in mouse models and on skin samples from systemic sclerosis patients suggests these drugs pose a promising area of future translational…
In a new study, Spiera et al. assessed the safety and efficacy of lenabasum, a synthetic, orally administered agonist of cannabinoid receptor 2 that modulates the endocannabinoid system to activate the resolution phase of innate immune responses, in diffuse cutaneous systemic sclerosis…

Patients with systemic sclerosis (SSc) may benefit from targeted stroke screening or prevention therapies. A recent study revealed SSc may be independently associated with stroke, finding the risk of stroke was 20–30% higher in SSc patients than healthy controls…

Michael Putman, MD |
The U.S. Food & Drug Administration (FDA) approved nintedanib for systemic sclerosis associated interstitial lung disease (SSc-ILD) on Sept. 6 after a randomized, controlled trial (SENSCIS) demonstrated significant benefit against placebo.1 At a cost of $96,000 per year, treatment reduced the adjusted annual rate of change in forced vital capacity (FVC) from –93.3 mL in…

A recent study investigated the possible differences in nailfold videocapillaroscopy in four types of inflammatory myopathies. Researchers observed giant capillaries, disorganization and major capillary loss in dermatomyositis and overlap myositis patients, finding dermatomyositis and overlap myositis imaging was different from that of antisynthetase syndrome and immune-mediated necrotizing myopathy…

A prognostic tool developed to predict survival in patients with various forms of pulmonary arterial hypertension (PAH) is fairly accurate in predicting survival outcomes for many patients with PAH related to systemic sclerosis (SSc-PAH), according to a new study. However, the prognostic accuracy is less reliable for SSc-PAH patients with the highest risk of death….
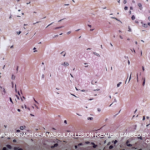
Wesam Gouda, MBBCh, MSc; Abdelhafeez Moshrif, MBBCh, MSc, MD; Fatma H. El Nouby, MBBCh, MSc, MD; & Amal Fehr, MBBCh, MSc, MD |
Systemic sclerosis (SSc) is a multi-system connective tissue disease in which skin and internal organ fibrosis are associated with an obliterative micro-vasculopathy and a degree of inflammation.1 Patients often report it takes one to three years from the appearance of the first signs and symptoms before they receive a diagnosis. The signs and symptoms of…
In September 2018, the U.S. Food & Drug Administration (FDA) granted fast-track status to FCX‑013, a gene therapy product developed to treat moderate to severe localized scleroderma (morphea). Previously, the treatment received an orphan drug designation for localized scleroderma, as well as a rare pediatric disease designation. Phase 1 and 2 studies will assess safety…