Soon, rheumatologists may have another drug to offer their patients with refractory rheumatoid arthritis (RRA) for whom effective and safe treatment remains challenging.
A study published in the New England Journal of Medicine shows that patients with RRA treated with once-daily baricitinib in a 4 mg dose had a significant clinical improvement in symptoms of active disease compared with placebo.1
“Baricitinib was effective in the treatment of patients with active rheumatoid arthritis,” says lead author of the study, Mark C. Genovese, MD, the James W. Raitt MD Professor, Department of Medicine, Division of Immunology & Rheumatology, Stanford University Medical School, Stanford, Calif. “Importantly, it demonstrated efficacy in a population of patients who had previously failed to adequately respond to many other currently available conventional and biologic therapies.”
The Study
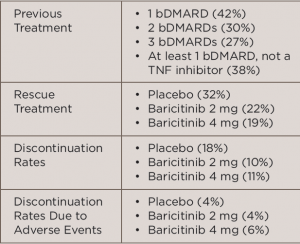
In the international, multicenter, Phase 3 study, called the RA-BEACON trial, 527 patients with RRA were randomized to receive 2 mg baricitinib daily (n=174), 4 mg baricitinib daily (n=177) or placebo (n=176) for 24 weeks. All patients were at least 18 years old, had moderate to severe active RA (≥6 tender joints of 68 joints examined and serum C-reactive protein level ≥3 mg/L) and had an inadequate response to, or unacceptable side effects with, at least one prior treatment with a tumor necrosis factor (TNF) inhibitor, other biologic disease-modifying anti-rheumatic drug (bDMARD) or both. Table 1 (below) lists treatment-related characteristics.
(click for larger image)
TABLE 1: Treatment-Related Characteristics
Key: bDMARD, biologic disease-modifying anti-rheumatic drugs; TNF, tumor necrosis factor
The primary endpoint of the study was the proportion of patients who had an ACR20 response at 12 weeks, with the primary comparison between patients treated with baricitinib 4 mg daily and placebo.
Based on the primary endpoint, the study found that significantly more patients treated with 4 mg baricitinib had an ACR20 response at 21 weeks compared with placebo (55% vs. 27%, P<0.001).
The study also assessed secondary endpoints, including ACR50 and ACR70 responses, physical function as measured by the Health Assessment Questionnaire-Disability Index (HAQ-DI) and disease activity as measured by the 28-joint Disease Activity Score (DAS). The last measure is based on the level of high-sensitivity C-reactive protein (DAS28-CRP) or the erythrocyte sedimentation rate (DAS28-ESR), the Clinical Disease Activity Index (CDAI) and the Simplified Disease Activity Index (SDAI).
Based on these endpoints, the study found that patients treated with 4 mg baricitinib also had significant improvements compared with placebo in DAS28-CRP and HAQ-DI (<0.001 for both measures). No significant difference between the two groups was seen for SDAI.
Results
Commenting on these results, Ziv Paz, MD, an instructor in medicine at the Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, emphasized the importance of these results in treating a very challenging population of patients.


