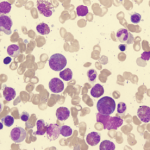
Eosinophilia is usually defined as an eosinophil count of more than 500/microL in peripheral blood.1 An eosinophil count of more than 1,500 is referred to as hypereosinophilia (HE); hypereosinophilic syndrome (HES) is defined as HE associated with organ dysfunction attributable to eosinophilia.2
Eosinophilia can occur due to infectious, malignancy, autoimmune or allergic etiologies. However, a significant number of patients do not have any identifiable etiology and are classified as idiopathic hypereosinophilic disorder, or hypereosinophilic disorder of unknown significance when there’s no end organ involvement.
Numerous stem cell mutations have been proposed to be associated with primary/idiopathic HE conditions.3,4 Heterogeneity of eosinophilic disorders can often make diagnosis challenging.
Below, we present a case of hypereosinophilic disorder that underscores the importance of detailed history and physical examination in the evaluation of hypereosinophilia.
Case Presentation
A 76-year-old Caucasian woman was admitted to our tertiary care hospital with diffuse pruritic morbilliform rash and progressive worsening of shortness of breath for six months. The rash onset was preceded by a trip to Napa Valley, Calif., and a prolonged course of upper respiratory tract infection. The patient’s past medical history was significant for childhood onset stable asthma and a recent diagnosis of chronic obstructive pulmonary disease associated with chronic smoking.
Prior to admission, her work-up revealed an eosinophil count of 700/microL (normal = 0–400/microL), C-reactive protein (CRP) at 9.7 mg/L (normal = 0–4.9 mg/L), a sedimentation rate (ESR) of 37 mm/HR, an anti-nuclear antibody (ANA) level of 1:320 titer in nucleolar pattern (normal = <1:160 titer), a cyclic citrullinated peptide (CCP) antibody level of 38 units (normal = 0–19 units), positive perinuclear antineutrophil cytoplasmic (pANCA) titer of 1:80 (normal = <1:20) and ), negative anti-myeloperoxidase antibody (anti-MPO). She underwent two skin biopsies prior to hospital admission, both suggestive of allergic contact dermatitis. She had tried a moderate dose of prednisone with minimal symptom relief.
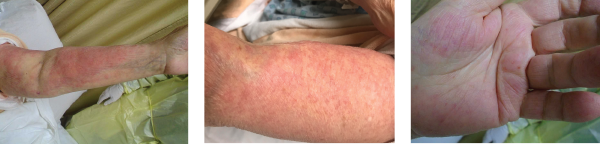
Images 1, 2 & 3: The patient’s examination revealed a diffuse morbilliform rash.
She was advised to consult with an infectious disease consultant for evaluation of a persistent rash, peripheral eosinophilia and dyspnea on exertion. At the time of infectious disease consultation, her rash had continued to worsen, and she was hypoxemic. Therefore, hospital admission was recommended.
At the time of hospital admission, the patient’s eosinophil count measured 2,200/uL, and her immunoglobulin E was within normal limits. (She was not on prednisone because a bone-marrow biopsy was planned. She was started on prednisone following the biopsy, which reduced her eosinophil count to 700/uL.) During hospitalization, an extensive workup included blood cultures, Toxocara serology, Coccioides serology, Aspergillosis serology, ova and parasite examination of stool (serology was requested), and an enteric parasite panel by PCR—all of which proved unremarkable. The patient underwent a hip X-ray for hip pain, and it revealed soft tissue calcifications in her left proximal femur. A computed tomography (CT) of her pelvis was performed to further evaluate the lesions seen in X-ray; the imaging suggested circumscribed calcification in the vastus lateralis muscle and atrophic uterus. An echocardiogram revealed normal cardiac function.
Some pathogens responsible for travelers’ diarrhea, such as dientamoeba fragilis, isospora belli & sarcocystis species, can cause eosinophilia. Fungal infections, such as aspergillosis, histoplasmosis, cryptococcosis & coccidiomycosis, often feature associated eosinophilia.
Bone marrow biopsy was also requested to evaluate for myeloproliferative or lymphocytic etiologies for HES, and it showed normocellular marrow with maturing trilineage hematopoiesis. Flow cytometric analysis performed on a single cell suspension prepared from bone marrow aspirate revealed no monoclonal B cell population or aberrant T cell phenotype. Fluorescence in-situ hybridization (FISH) analysis was negative for a deletion at 4q12 (including CHIC2) and for fusion of the FIP1L1 and PDGFRA genes, a rearrangement involving PDGFRB, or a BCR/ABL1 rearrangement.