Although the true cause of systemic sclerosis (SSc), or scleroderma, remains unknown, researchers have made progress in detecting the autoimmune disease’s early presence. Beyond the physiological signs of Raynaud’s phenomenon, a capillaroscopy can detect alterations in microcirculation and lab tests can confirm the presence of telltale autoantibodies, such as anti-topoisomerase 1, anti-centromere and anti-RNA polymerase III, in serum.
Later symptoms may include vascular abnormalities with progressing fibrosis in the upper and lower gastrointestinal tract, kidneys and lungs.
“When the disease progresses, you can get the classical tissue involvement and symptoms beyond skin involvement,” says Maurizio Cutolo, MD, professor of rheumatology and internal medicine and director of research laboratories in the Academic Division of Clinical Rheumatology at the University of Genova, Italy. “But this is a story of cells that precede these changes.”
Researchers would dearly love to clarify the identities and alterations occurring early on in these cells. Most studies, though, haven’t examined the characteristics of immune cells past the involvement of B lymphocytes. Of those that have, many suggest that SSc monocytes and macrophages display a phenotype most closely associated with the anti-inflammatory M2 activation state.
Two recent studies in the Annals of the Rheumatic Diseases, including one by Dr. Cutolo’s research group, used different flow cytometry methods to take a much closer look. Their joint conclusion is that from the perspective of macrophage polarization, the SSc picture may be far more complicated than previously assumed.1,2 In fact, they suggest SSc cells may be much better characterized as a hybrid between the M2 and pro-inflammatory M1 polarization states.
Taking a Closer Look
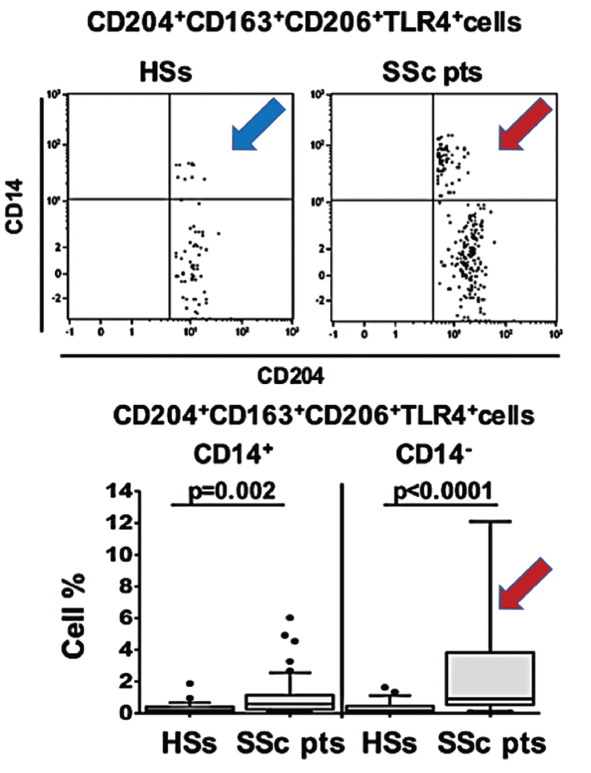
(click for larger image) Figure 1. In this figure, arrows show the increased population of blood circulating hybrid M1/M2 macrophages in scleroderma patients (red SSc) compared with healthy matched subjects (blue HSs).
As progenitor cells, circulating monocytes change in response to physical contact with other cells and signals from cytokines, growth factors and other soluble mediators in the microenvironment. When they differentiate into macrophages, the cells can be pulled toward the M1 activation state typical of immune defense and rheumatoid arthritis, or the M2 state more characteristic of wound healing, immune suppression, fibrosis and cancer growth and proliferation.
Polarization toward M1 or M2 can be identified via the expression levels of associated cell surface markers. The new studies suggest these blood-based biomarkers could help define the disease state. In addition, the high concentrations of hybrid cells circulating in the blood prior to their homing and specialization in body tissues might offer a promising target for early treatment.
“The emerging picture is that now we are investigating the role of not just B cells, but also the monocytes and macrophages in systemic sclerosis,” Dr. Cutolo says. “Now. we know they play an important role as cells involved in the fibrotic process.”
Dr. Cutolo emphasizes that such potential applications should be verified. Even so, he notes a subsequent study by his group suggested systemic sclerosis patients with interstitial lung disease have high concentrations of the M1/M2 hybrid monocyte/macrophage population as well.3
Collectively, Dr. Cutolo says, the research points toward a better way to characterize SSc patients, particularly when the disease involves the lungs. Beyond their utility as biomarkers of disease, the cell surface markers could be further examined for their role in pathogenesis. “So we have in our hands a lot of work to do, but they are very promising results, very interesting results,” he says.
Patricia Pioli, PhD, an assistant professor of microbiology and immunology at Dartmouth University who wasn’t involved in either study, agrees the research represents a big step forward in characterizing SSc. “These studies are really important in that they’re the first time, really, that people are looking at immune cells—macrophages and monocytes—in the context of the disease and trying to characterize them on some level beyond saying that they’re there,” she says.
The take-home message, Dr. Pioli says, is that there’s nothing uniform about the mix of activated macrophages in the disease. “Data from our lab in this regard also indicate that in the context of scleroderma, macrophages are not uniformly conforming to this either M1 or M2 activation profile,” she says. Therefore, rheumatologists should take a closer look at these peripheral blood cell populations and not just fibroblasts.

