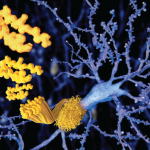
Most healthcare professionals do not frequently encounter, or are even actively aware of, amyloid protein–related disorders beyond the most commonly known form, Alzheimer’s disease, which is caused by the aggregation of beta-amyloid proteins in the brain. However, for rheumatologist Jonathan Kay, MD, professor of medicine at the University of Massachusetts Medical School in Boston, his awareness of, and subsequent focus on the wider world of amyloidosis stemmed from a seemingly unrelated patient referral.
“I saw my first case of amyloidosis when a nephrologist colleague sent me a dialysis patient who was experiencing carpal tunnel syndrome,” he recalled. Fortunately for both Dr. Kay and his new patient, Dr. Kay had just read a newly published paper on the topic of dialysis-related amyloidosis (where beta 2 microglobulin is the causative protein subunit) so he was not only able to help his patient by providing her with a neutral-resting wrist splint, but he also inadvertently expanded his population of potential patients.1 “She was very pleased with the results and went back to her nephrologist and told him. Pretty soon, the average serum creatinine level of patients in my rheumatology clinic was around 10 mg/dL because of all the referred dialysis patients.”
Dr. Kay has since become an expert on the topic of amyloidosis in general, and recently shared his observations at the 2012 meeting of the American College of Rheumatology, held last November in Washington, D.C.
Amyloidosis Defined
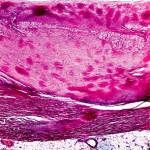
In essence, amyloidosis is a disease caused by protein misfolding. Once expressed and then misfolded, these proteins infiltrate, aggregate, and then form insoluble fibrils that interfere with the normal function of a number of vital organs.
There are at least 18 endogenous human proteins that can be involved in the pathogenesis of distinct types of amyloidosis, each of which forms fibrils in combination with various members of an array of glycosaminoglycans. However, most types of amyloidosis share a specific component—serum amyloid P protein—a critical bit of information when considering imaging modalities for amyloidosis, as discussed below.
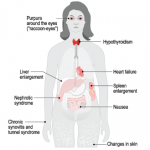
Once a nucleus of misfolded proteins forms, it serves as a template for further aggregation, thereby driving the progression of a given amyloidosis disease state.2 The organs/systems involved may include the brain, heart, kidneys, gastrointestinal tract, liver, spleen, peripheral nervous system, and musculoskeletal system.
Types of amyloidosis are grouped as localized, relative to a specific organ (e.g., Alzheimer’s), or systemic. Systemic forms of amyloidosis are further delineated by cause. For primary (AL) amyloidosis, the most common form of the disease, the subunit protein is the immunoglobulin light chain produced in bone marrow. Secondary, or acquired, (AA) amyloidosis typically results from chronic infection or inflammation. This form is rarely seen in the U.S., probably because of the availability of effective antiinflammatory and antibiotic treatments. The amyloidosis that developed in dialysis patients arose because of increased production and inadequate clearance of the subunit protein, β2-microglobulin (Aβ2M). Amyloidosis may also be hereditary in origin, resulting from mutations of transthyretin (ATTR) and other subunit proteins.