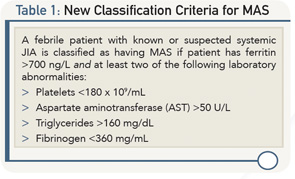
BOSTON—In a session on the role of macrophages in rheumatic diseases, Macrophages Gone Wild, at the ACR/ARHP Annual Meeting in Boston, November 2014, a panel of experts walked participants through the pathogenesis of and new classification criteria for macrophage activation syndrome (MAS), a severe complication of rheumatic diseases characterized by inflammatory multi-organ failure and hyperferritinemia. Although the complication occurs primarily in the setting of systemic juvenile idiopathic arthritis (sJIA), the presence of MAS outside of this setting was also discussed.
Pathogenesis of MAS
Although MAS, in part, got its name from the thought that hemophagocytosis is a primary pathological driver of the disease, Edward M. Behrens, MD, Joseph Lee Hollander Chair in Pediatric Rheumatology, chief, Division of Rheumatology, The Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, opened the session saying that hemophagocytosis is emerging as less of a driver than previously thought.

As such, he prefers calling this syndrome “cytokine storm syndrome” because of the multiple cytokines being spewed out by the immune systems that lead to systemic inflammatory response caused by the environment, genetic factors or combined factors.
Focusing on genetic-related MAS, he talked about the common pathogenesis of MAS and familial hemophagocytic lymphohistiocytosis (FHL). Important for the pathogenesis of both MAS and FHL, he said, is a combination of both defects in cytotoxic cell killing, as well as cytokine effects. Key cytokines involved in the pathogenesis include numerous proinflammatory interleukins (ILs), including IL-6, IL-4, IL-1/18 and IL-33, as well as interferon-gamma.
‘We need to recognize what’s been happening in the past, but we need to stop wasting time & dollars on unimportant research.’
He also emphasized that despite the similarities between MAS and FHL, their fundamental pathoetiologies are likely to be different. For example, in FHL, a defect in cytotoxic granule release from CD8+ T cells is a necessary factor, but insufficient for disease development. In MAS, some cases may be due in part to defective cytotoxic granule function, but inflammation-induced cytokines modify or completely supplant this effect.
Although this moves us to a closer understanding of the pathogenesis of MAS, Dr. Behrens emphasized that more work needs to be done to understand how inflammation-induced cytokines may modify the effect of defective cytotoxic granule function on the development of MAS. Potential areas of inquiry include whether hypercytokinemia allow hypomorphic CTL function to reveal itself as MAS and which scenarios don’t require hypomorphic CTL function to turn into MAS.