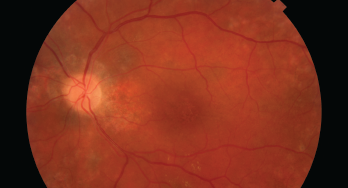
This image shows damage to the retina, which can be caused by HCQ.
Since 1991, hydroxychloroquine (HCQ) has been a staple for the treatment of patients with systemic lupus erythematosus; it has been shown to improve survival, reduce cardiovascular risk, thrombosis and renal damage, delay or prevent lupus cerebritis and more. However, HCQ can potentially bind in the retinal pigment epithelium and cause degeneration of photoreceptors, leading to retinopathy.
A recently released statement endorsed by the ACR, the American Academy of Ophthalmology (AAO), the American Academy of Dermatology (AAD) and the Rheumatologic Dermatology Society (RDS) underscores points of agreement about the management of HCQ with respect to possible eye toxicity.1 The statement emphasizes the need for effective communication among different specialty physicians and patients to optimize eye safety while ensuring appropriate patients can obtain this important drug.
Background: Perspectives from the AAO
Ocular toxicity from HCQ is of particular concern because it is untreatable, irreversible and progressive if caught at a late stage, even if the drug is stopped.
In 2016, the AAO used new data to update its previous recommendations on screening for chloroquine and HCQ retinopathy.2 Among its key advice was a proposed maximum daily dose of HCQ of no greater than 5.0 mg/kg/day (based on real body weight).
HCQ is packaged as 200 mg pills, and 400 mg per day has been a common dose prescribed by rheumatologists. But under the AAO recommendations, this standard dose was deemed too high for many patients (i.e., a dose of 400 mg per day would be acceptable for an average American man, but too much for the average American woman, based on weight).
Since then, rheumatologists have differed somewhat in their acceptance of these recommendations and have continued to debate the ideal dosing schedule. Some rheumatologists have continued to use doses up to 600 mg per day and/or up to 6.5 mg/kg per day, which was an earlier recommended target dose.3,4 In particular, some have emphasized its critical role for lupus, because HCQ is associated with multiple benefits for patients with this diagnosis.5
This is an issue not just for rheumatologists, but for dermatologists, who have used similar doses of HCQ for multiple applications.
Joint Statement
Ultimately, members of these medical societies agreed to write a shared statement that could be jointly endorsed.1 The working group composing the statement consisted of experts in potential HCQ toxicity: seven rheumatologists, two ophthalmologists and two dermatologists.


