 Baricitinib is a preferential JAK1 and JAK2 inhibitor that has completed Phase 2 clinical trials, the results of which were published in 2015.
Baricitinib is a preferential JAK1 and JAK2 inhibitor that has completed Phase 2 clinical trials, the results of which were published in 2015.
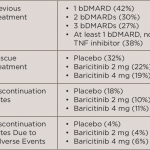
In a recent Phase 3 study, baricitnib was used to treat patients with rheumatoid arthritis (RA) who had an inadequate response to or unacceptable side effects from more than one tumor necrosis factor inhibitor or other biologic disease-modifying anti-rheumatic drug (DMARD).1 This study was the RA-BEACON trial, which was a randomized, 24-week, double-blind, placebo-controlled study in 24 countries and 178 centers.
Participants were randomized to receive 2 mg baricitinib daily, 4 mg baricitinib daily or placebo. Continued treatment with stable doses of conventional synthetic DMARDs, NSAIDs, analgesics, glucocorticoids (≤10 mg prednisone or equivalent) or a combination of these agents was allowed. At Weeks 14 and 16, patients with less than a 20% improvement in tender and swollen joints were given 4 mg baricitinib daily as a rescue medication. After Week 16, rescue medication was allowed at the investigators’ discretion. Patients completing the study could enter a long-term extension study or be evaluated 28 days later for follow-up. The primary endpoint was the achievement of ACR20 at Week 12; secondary endpoints included: the Health Assessment Questionnaire–Disability Index (HAQ-DI) score with a change from baseline, the 28-joint Disease Activity Score based on C-reactive protein level (DAS28-CRP) with a change from baseline and a Simplified Disease Activity Index (SDAI) score of <3.3 (on a scale of 0.1 to 86.0; a score of <3.3 indicates remission).
Five hundred and twenty-seven patients were randomized into groups with a 1:1:1 ratio. In the group treated with 4 mg baricitinib, significantly more patients achieved ACR20 compared with placebo (55% baricitinib vs. 27% placebo, P<0.001). The 4 mg baricitinib-treated patients also had significant responses for the HAQ-DI score and the DAS-28-CRP, but not for the SDAI <3.3. Of enrolled patients, 40–46% had received at least one previous biologic, 27–33% had received at least two biologics, and 25–29% of patients had received at least three biologic agents.
More patients treated with baricitinib compared with placebo experienced adverse events. Patients treated with 4 mg baricitinib had more infections (71–77% vs. 64% placebo), as well as those treated with 2 mg baricitinib (40–44% vs. 31% placebo). Serious adverse events occurred in 4% of 2 mg baricitinib-treated patients, 10% of 4 mg baricitinib-treated patients and 7% of placebo-treated patients. In the 4 mg baricitinib-treated group, two participants had nonmelanoma skin cancers, and two had major cardiovascular events, including a fatal stroke. Small reductions in neutrophil counts, small increases in serum creatinine and small increases in low-density lipoprotein levels occurred with baricitinib.